Abstract
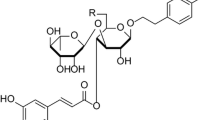
In order to improve the production of the cytotoxic lignan podophyllotoxin, seven precursors from the phenylpropanoid-routing and one related compound were fed to cell suspension cultures derived from the rhizomes of Podophyllum hexandrum Royle. These cell cultures were able to convert only coniferin into podophyllotoxin, maximally a 12.8 fold increase in content was found. Permeabilization using isopropanol, in combination with coniferin as a substrate, did not result in an extra increase in podophyllotoxin accumulation. Concentrations of isopropanol exceeding 0.5% (v/v) were found to be rather toxic for suspension growth cells of P. hexandrum. When coniferin was fed in presence of such isopropanol concentrations, β-glucosidase activity was still present, resulting in the formation of the aglucon coniferyl alcohol. In addition, podophyllotoxin was released into the medium under these permeabilization conditions. Entrapment of P. hexandrum cells in calcium-alginate as such or in combination with the feeding of biosynthetic precursors, did not improve the podophyllotoxin production. Cell-free medium from suspension cultures at later growth stages incubated with coniferin, resulted in the synthesis of the lignan pinoresinol.
Similar content being viewed by others
References
Van Maanen JMS, Retèl J, De Vries J & Pinedo HM (1988) Mechanism of action of antitumor drug etoposide: a review. J. Natl. Cancer. Inst. 80: 1526–1533
Holthuis JJM (1988) Etoposide and teniposide. Bioanalysis, metabolism and clinical pharmokinetics. Pharm. Weekbl. (Sci.) 10: 101–116
Pelter A (1986) Lignans: Some properties and syntheses. Rec. Adv. Phytochem. 20: 201–241
MacRae WD & Towers GHN (1984) Biological activities of lignans. Phytochemistry 23: 1207–1220
Jackson DE & Dewick PM (1984) Aryltetralin lignans from Podophyllum hexandrum and Podophyllum peltatum. Phytochemistry 23: 1147–1152
Chuang M & Chang WC (1987) Embryoid formation and plant regeneration in callus cultures derived from vegetative tissues of Dysosma pleianthum (Hance) Woodson. J. Plant Physiol. 128: 279–283
Rust RW & Roth RR (1981) Seed production and seedling establishment in the mayapple. Podophyllum peltatum L. Am. Midl. Natur. 105: 51–60
Van Uden W, Pras N, Visser JF & Malingré ThM (1989) Detection and identification of podophyllotoxin produced by cell cultures derived from Podophyllum hexandrum Royle. Plant Cell Reports 8: 165–168
Rhodes MJC (1985) Immobilized plant cell cultures. In: Wiseman A (Ed) Topics in Enzyme and Fermentation Biotechnology 10 (pp 51–87). Ellis Horwood Limited, Chichester
Brodelius P & Mosbach K (1982) Immobilized plant cells. General aspects. J. Chem. Tech. Biotechnol. 32: 330–337
Hall RD, Holden MA & Yeoman MM (1988) Immobilization of higher plant cells. In: Bajaj YPS (Ed.) Biotechnology in Agriculture and Forestry 4. Medicinal and Aromatic Plants. I (pp 136–156). Springer-Verlag Berlin, Heidelberg, New York
Wichers HJ, Maligré ThM & Huizing HJ (1983) The effect of some environmental factors on the production of L-DOPA by alginate-entrapped cells of Mucuna pruriens. Planta 158: 482–486
Pras N, Wichers HJ, Bruins AP & Malingré ThM (1988) Bioconversion of para-substituted monophenolic compounds into corresponding catechols by alginate entrapped cells of Mucuna pruriens. Plant Cell Tiss. Org. Cult. 13: 15–26
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Maehly AC & Chance B (1954) The assay of catalases and peroxidases. In: Glick D (Ed.) Methods of Biochemical Analysis. Volume I (pp 357–424). Interscience Publishers, Inc. New York
Luckner M (1986) Secondary metabolism in microorganisms, plants and animals, 2nd ed. (pp 437–444). Springer-Verlag Heidelberg, New York, Tokyo
Freudenberg K & Harkin JM (1963) The glucosides of cambial sap of spruce. Phytochemistry 2: 189–193
Hahlbrock K (1977) Regulatory aspects of phenylpropranoid biosynthesis in cell cultures. In: Barz W, Reinhard E & Zenk MH (Eds) Proceedings in Life Sciences. Plant Tissue Culture and its Bio-technological Application (pp 95–111). Springer-Verlag Berlin, Heidelberg, New York
Stöckigt J & Klischies M (1977) Biosynthesis of lignans. Part I. Biosynthesis of artiin and phyllyrin. Holzforschung 31: 41–44
Berlin J, Martin B, Nowak J, Witte L, Wray V & Strack D (1989) Effects of permeabilization on the biotransformation of phenylalanine by immobilized tobacco cell cultures. Z. Naturforsch 44c: 249–254
Pras N, Hesselink PGM, Ten Tusscher J & Malingré ThM (1989) Kinetic aspects of the bioconversion of L-tyrosine into L-DOPA by cells of Mucuna pruriens L. entrapped in different matrices. Biotechnol. Bioeng. 34: 214–222
Sticher L, Penel C & Greppin H (1981) Calcium requirement for the secretion of peroxidases by plant cell suspensions. J. Cell Sci. 48: 345–353
Duffield AM (1967) Mass spectrometric fragmentation of some lignans. J. Heterocyclic. Chem. 4: 16–22
Hösel W & Todenhagen R (1980) Characterization of β-glucosidase from Glycine max which hydrolyses coniferin and syringin. Phytochemistry 19: 1349–1353
Erdtman H (1955) Lignans. In: Peach K & Tracey MV (Eds) Modern Methods of Plant Analysis (pp 428–449). Springer-Verlag, Berlin, Heidelberg, New York
Freudenberg K & Rasenack D (1953) d,1-Pinoresinol, ein weiteres Zwischenprodukt der Ligninbildung. Chem. Ber. 6: 756–758
Harborne JB (1980) Plant phenolics. In: Bell EA & Charlwood BV (Eds) Encyclopedia of Plant Physiology. New series volume 8. Secondary Plant Products (pp 329–402). Springer-Verlag, Berlin, Heidelberg, New York
Jackson DE & Dewick PM (1984) Biosynthesis of Podophyllum lignans-II. Interconversions of aryltetralin lignans in Podophyllum hexandrum. Phytochemistry 223: 1037–1042
Ayres DC (1969) Incorporation of L-[U-14C]-β-phenylalanine into the lignan podophyllotoxin. Tetrahedron Lett. 11: 883–886
Ayres DC, Farrow A & Carpenter BG (1981) Lignans and related phenols. Part 16. The biogenesis of podophyllotoxin. J. Chem. Soc. Perkin Trans. I: 2134–2136.
Jackson DE & Dewick PM (1984) Biosynthesis of Podophyllum lignans. I. Cinnamic acid precursors of podophyllotoxin in Podophyllum hexandrum. Phytochemistry 23: 1029–1035
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Uden, W., Pras, N. & Malingré, T.M. On the improvement of the podophyllotoxin production by phenylpropanoid precursor feeding to cell cultures of Podophyllum hexandrum Royle. Plant Cell Tiss Organ Cult 23, 217–224 (1990). https://doi.org/10.1007/BF00034435
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034435