Abstract
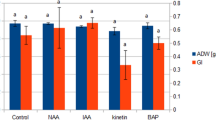
The formation of 5 hairy root lines of Leontopodium alpinum was induced by infection of sterile plants with Agrobacterium rhizogenes. The transformed roots were grown as batch cultures in a phytohormone-free modified Murashige & Skoog medium. A time-course experiment with the most productive line showed that a culture period of 6 weeks was optimum for biomass production yielding a 70-fold increase in fresh weight. A 70% enhancement of anthocyanin formation could be induced by addition of benzyladenine (to a final concentration of 0.5 mg l-1) to the culture medium 14 days before harvest. The presence in the cultures of chlorogenic acid as well as other hydroxycinnamic acid esters was confirmed by TLC. An essential oil (ca. 0.6%) was separated from the hairy roots by steam distillation, a high variability in oil yield being observed between the different lines. GC analyses showed the oils to be complex mixtures of > 30 compounds, with 2 of these consistently representing ca. 60% of the oils. The essential oils isolated from hairy roots were found to be qualitatively similar to the natural root oil, although quantitative differences in oil components were apparent. Oil yields could be increased by growing roots in the absence of light.
Similar content being viewed by others
Abbreviations
- MS:
-
Murashige & Skoog
- BAP:
-
benzylaminopurine
References
Anonymous (1980) British Pharmacopoeia, Vol II, Appendix XI. E. HMSO, London
Bicci C, Nano GM & Tira S (1975) n-Paraffin components of some Gnaphalieae. Planta Med. 28: 389–391
Bokadia MM, MacLeod AJ, Mehta SC, Mehta BK & Patel H (1986) The essential oil of Inula racemosa. Phytochemistry 25: 2887–2888
Comey N, Hook I & Sheridan H (1992a) Essential oil from normal and hairy roots of Leontopodium alpinum. Proceedings of 23rd International Symposium on Essential Oils, Ayr, Scotland
Comey N, Hook I & Sheridan H (1992b) Enhancement of anthocyanin production in cell cultures and hairy roots of Leontopodium alpinum. Planta Med. 58, Suppl. 1: A 605
Hashimoto T & Yamada Y (1991) Organ Culture and Manipulation. In: Hostettmann K (Ed) Methods in Plant Biochemistry, Vol. 6. Assays for Bioactivity (pp 323–350). Academic Press, London
Hennessy D, Hook I, Sheridan H & McGee A (1989) Hydroxycinnamic acid esters from cell suspension cultures and plants of Leontopodium alpinum. Phytochemistry 28: 489–490
Hook I (1993) Leontopodium alpinum Cass. (Edelweiss): In vitro culture and production of secondary metabolites (Ch. XV). In: Bajaj YPS (Ed) Biotechnology in Agriculture and Forestry, Vol 21. Medicinal and Aromatic Plants IV (pp 217–232). Springer-Verlag, Heidelberg
Hu ZB & Alfermann AW (1983) Diterpenoid production in hairy root cultures of Salvia miltirrhiza. Phytochemistry 32: 699–703
Jaziri M, Legros M, Homes J & Vanhaelen M (1988) Tropine alkaloids production by hairy root cultures of Datura stramonium and Hyoscyamus niger Phytochemistry 27: 419–420
Mulder-Krieger Th, Verpoorte R, Svendsen AB & Scheffer JJC (1988) Production of essential oils and flavours in plant cell and tissue cultures. A review. Plant Cell Tissue Organ Cult. 13: 85–154
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Parr AJ & Hamill JD (1987) Relationship between Agrobacterium rhizogenes transformed hairy roots and intact, infected Nicotiana plants. Phytochemistry 26: 3241–3245
Payne J, Hamill JD, Robbins RJ & Rhodes MIC (1987) Production of hyoscyamine by ‘hairy’ root cultures of Datura stramonium. Planta Med. 53: 474–478
Saito K, Yamazaki M & Murakoshi I (1992) Transgenic medicinal plants: Agrobacterium mediated foreign gene transfer and production of secondary metabolites. J. Nat. Prod. 55: 149–162
Sauerwein M & Shimomura K (1991) Alkaloid production in hairy roots of Hyoscyamus albus transformed with Agrobacterium rhizogenes. Phytochemistry 30: 3277–3280
Tira S, Galeffi C & Di Modica G (1970) Flavonoids of Gnaphalieae: Leontopodium alpinum. Experientia 26: 1192
Trotin F, Moumou Y & Vasseur J (1993) Flavanol production by Fagopyrum esculentum hairy and normal root cultures. Phytochemistry 32: 929–931
Wagner H, Bladt S & Zgainski EM (1984) Plant Drug Analysis. Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hook, I. Secondary metabolites in hairy root cultures of Leontopodium alpinum Cass. (Edelweiss). Plant Cell Tiss Organ Cult 38, 321–326 (1994). https://doi.org/10.1007/BF00033892
Issue Date:
DOI: https://doi.org/10.1007/BF00033892