Abstract
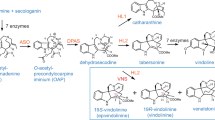
The enzyme, desacetoxyvindoline 4-hydroxylase, was purified to apparent homogeneity from Catharanthus roseus by ammonium sulfate precipitation and successive chromatography on Sephadex G-100, green 19-agarose, hydroxylapatite, α-kg sepharose and Mono Q. The 4-hydroxylase was characterized by its strict specificity for position 4 of desacetoxyvindoline suggesting it to catalyze the second to last step in vindoline biosynthesis. The molecular mass of the native and denatured 4-hydroxylase was 45 kDa and 44.7 kDa, respectively, suggesting that the native enzyme is a monomer. Two-dimensional isoelectric focusing under denaturing conditions resolved the purified 4-hydroxylase into three charge isoforms of pIs 4.6, 4.7 and 4.8. The purified 4-hydroxylase exhibited no requirement for divalent cations, but inactive enzyme was reactivated in a time-dependent manner by incubation with ferrous ions. The enzyme was not inhibited by EDTA or SH-group reagents at concentrations up to 10 mM. The mechanism of action of desacetoxyvindoline 4-hydroxylase was investigated. The results of substrate interaction kinetics and product inhibition studies suggest an Ordered Ter Ter mechanism where α-kg is the first substrate to bind followed by the binding of O2 and desacetoxyvindoline. Their K m values for α-kg, O2 and desacetoxyvindoline are 45 μM, 45 μM and 0.03 μM, respectively. The first product to be released was deacetylvindoline followed by CO2 and succinate, respectively.
Similar content being viewed by others
Abbreviations
- α-kg:
-
α-ketoglutarate or 2-oxoglutarate
- NMT:
-
N-methyltransferase
- SAM:
-
S-adenosyl-l-methionine
- TLC:
-
thin layer chromatography
- VBL:
-
vinblastine
- VCR:
-
vincristine
References
Balsevich J, De Luca V & Kurz WGW (1986) Altered alkaloid pattern in dark grown seedlings of Catharanthus roseus. The isolation and characterization of 4-desacetoxyvindoline: a novel indole alkaloid and proposed precursor of vindoline. Heterocycles 24: 2415–2421
Cleland WW (1967) Steady state kinetics. In: Boyer PD (Ed) The Enzymes. Vol II (pp 1–61) Academic Press, New York
Cleveland DW, Fisher SG, Kirscher MW & Laemmli UK (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis of gel electrophoresis. J. Biol. Chem. 252: 1102–1106
De Carolis E, Chan F, Balsevich J & De Luca V (1990) Isolation and characterization of a 2-oxoglutarate-dependent dioxygenase involved in the second last step in vindoline biosynthesis. Plant Physiol. 94: 1323–1329
De Carolis E & De Luca V (1993) Purification, characterization and kinetic analysis of a 2-oxoglutarate-dependent dioxygenase involved in vindoline biosynthesis from Catharanthus roseus. J. Biol. Chem. 268: 5504–5511
De Luca V (1993) Enzymology of indole alkaloid biosynthesis. In: Lea PJ (Ed) Methods in Plant Biochemistry, Vol 9 (pp 345–368), Academic Press, New York
De Luca V & Kurz WGW (1988) Monoterpene indole alkaloids (Catharanthus alkaloids). In: Constable F & Vasil IK (Eds) Cell Culture and Somatic Genetics of Plants, Vol 5 (pp 385–401). Academic Press, San Diego
Dethier M & De Luca V (1993) Partial purification of an N-methyltransferase involved in vindoline biosynthesis in Catharanthus roseus. Phytochemistry 32: 673–678
Holme E (1975) A kinetic study of thymine 7-hydroxylase from Neurospora crassa Biochemistry 14: 4999–5003
Myllyla R, Tuderman L & Kivirikko KI (1977) Mechanism of prolyl hydroxylase reaction: kinetic analysis of the reaction sequence. Eur. J. Biochem. 80: 349–357
Power R, Kurz WGW & De Luca V (1990) Purification and characterization of acetylcoenzyme A: deacetyvindoline 4- acetyltransferase from Catharanthus roseus. Arch. Biochem. Biophys. 279: 370–376
Puistola U, Turpeenniemi-Hujanen TM, Myllyla R & Kivirikko KI (1980) Studies on the lysyl hydroxylase reaction: Inhibition kinetics and the reaction mechanism. Biochim. Biophys. Acta 611: 51–60
Rhoads RE & Udenfriend S (1968) Decarboxylation of α-ketoglutarate coupled to collagen proline hydroxylase. Proc. Natl. Acad. Sci. 60: 1473–1478
Robinson T (1981) General theories of alkaloid biosynthesis. In: Kleinzeller A, Springer GF & Wittmann HG (Eds) The biochemistry of alkaloids: Molecular biology biochemistry and biophysics. Vol II (pp 12–136). Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Carolis, E., De Luca, V. A novel 2-oxoglutarate-dependent dioxygenase involved in vindoline biosynthesis: characterization, purification and kinetic properties. Plant Cell Tiss Organ Cult 38, 281–287 (1994). https://doi.org/10.1007/BF00033888
Issue Date:
DOI: https://doi.org/10.1007/BF00033888