Abstract
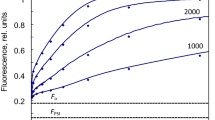
In order to characterize the photosystem II (PS II) centers which are inactive in plastoquinone reduction, the initial variable fluorescence rise from the non-variable fluorescence level Fo to an intermediate plateau level Fi has been studied. We find that the initial fluorescence rise is a monophasic exponential function of time. Its rate constant is similar to the initial rate of the fastest phase (α-phase) of the fluorescence induction curve from DCMU-poisoned chloroplasts. In addition, the initial fluorescence rise and the α-phase have the following common properties: their rate constants vary linearly with excitation light intensity and their fluorescence yields are lowered by removal of Mg++ from the suspension medium. We suggest that the inactive PS II centers, which give rise to the fluorescence rise from Fo to Fi, belong to the α-type PS II centers. However, since these inactive centers do not display sigmoidicity in fluorescence, they thus do not allow energy transfer between PS II units like PS IIα.
Similar content being viewed by others
Abbreviations
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethyl urea
- DMQ:
-
2,5-dimethyl-p-benzoquinone
- Fo :
-
initial non-variable fluorescence yield
- Fm :
-
maximum fluorescence yield
- Fi :
-
intermediate fluorescence yield
- PS II:
-
photosystem II
- QA :
-
primary quinone acceptor of PS II
- QB :
-
secondary quinone acceptor of PS II
References
Anderson JM and Melis A (1983) Localization of different photosystems in separate regions of chloroplast membranes. Proc Natl Acad Sci USA 80: 745–749
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Poly-phenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15
Barber J (1980) An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem I. FEBS Lett 118: 1–10
Bell DH and Hipkins MF (1985) Analysis of fluorescence induction curves from pea chloroplasts. Photosystem II reaction center heterogeneity. Biochim Biophys Acta 807: 255–262
Black MT, Brearley TH and Horton P (1986) Heterogeneity in chloroplast photosystem II. Photosynth Res 8: 193–207
Cao J and Govindjee (1990) Chlorophyll a fluorescence transient as an indicator of active and inactive photosystem II in thylakoid membranes. Biochim Biophys Acta 1015: 180–188
Chylla RA, Garab G and Whitmarsh J (1987) Evidence for slow turnover in a fraction of photosystem II complexes in thylakoid membranes. Biochim Biophys Acta 894: 562–571
Chylla RA and Whitmarsh J (1989) Inactive photosystem II complexes in leaves: Turnover rate and quantitation. Plant Physiol 90: 765–772
Chylla RA and Whitmarsh J (1990) Light saturation response of inactive photosystem II reaction centers in spinach. Photosynth Res 25: 39–48
Diner BA (1986) The reaction center of Photosystem II. In: Staehelin LA and Arntzen CJ (eds) Photosynthesis III, pp 422–436. New York: Springer-Verlag
Doschek WW and Kok B (1972) Photon trapping in photosystem II of photosynthesis. The fluorescence rise curve in in presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Biophys J 12: 832–838
Etienne AL (1974) Effects of carbonyl cyanide m-chlorophyenyl-hydrazone and hydroxylamine on the photosystem II electron exchange mechanism in DCMU-treated algae and chloroplasts. Biochim Biophys Acta 333: 497–508
Forbush B and Kok B (1968) Reaction between primary and secondary electron acceptors of photosystem II of photosynthesis. Biochim Biophys Acta 162: 243–253
Graan T and Ort DR (1986) Detection of oxygen-evolving photosystem II centers inactive in plastoquinone reduction. Biochim Biophys Acta 852: 320–330
Guenther JE and Melis A (1990) The physiological significance of photosystem II heterogeneity in chloroplasts. Photosynth Res 23: 105–109
Hodges M and Barber J (1986) Analysis of chlorophyll fluorescence induction kinetics exhibited by DCMU-inhibited thylakoid and the origin of α and β centers. Biochim Biophys Acta 848: 239–246
Horton P and Croze E (1979) Characterization of two quenchers of chlorophyll fluorescence with different midpoint oxidation-reduction potentials in chloroplasts. Biochim Biophys Acta 545: 188–201
Hsu BD, Lee YS and Jang YR (1989) A method for analysis of fluorescence induction curve from DCMU-poisoned chloroplasts. Biochim Biophys Acta 975: 44–49
Joliot P and Joliot A (1979) Comparative study of the fluorescence yield and of the C550 absorption change at room temperature. Biochim Biophys Acta 546: 93–105
Joliot P and Joliot A (1981) A photosystem II electron acceptor which is not a plastoquinone. FEBS Lett 134: 155–158
Lavergne J (1982a) Two types of primary acceptors in chloroplasts photosystem II. I. Different recombination properties. Photobiochem Photobiophys 3: 257–271
Lavergne J (1982b) Two types of primary acceptors in chloroplasts photosystem II. II. Reduction in two successive photoacts. Photobiochem Photobiophys 3: 273–285
Melis A (1985) Functional properties of photosystem II in spinach chloroplasts. Biochim Biophys Acta 808: 334–342
Melis A and Anderson JM (1983) Structural and functional organization of the photosystems in spinach chloroplasts, antenna size, relative electron-transport capacity and chlorophyll composition. Biochim Biophys Acta 724: 473–484
Melis A and Homann PH (1975) Kinetic analysis of the fluorescence induction in DCMU-poisoned chloroplast. Photochem Photobiol 21: 431–437
Melis A and Homann PH (1976) Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol 23: 343–350
Melis A and Ow RA (1982) Photoconversion kinetics of chloroplast photosystems I and II: Effect of Mg2+. Biochim Biophys Acta 682: 1–10
Mohanty P and Govindjee (1973) Light-induced changes in the fluorescence yield of chlorophyll a in Anacystis nidulans. I. Relationship of slow fluorescence changes with structural changes. Biochim Biophys Acta 305: 95–104
Sinclair J and Spence SM (1988) The analysis of fluorescence induction transients from dichlorophenyldimethylureapoisoned chloroplasts. Biochim Biophys Acta 935: 184–194
Sinclair J and Spence SM (1990) Heterogeneous photosystem 2 activity in isolated spinach chloroplasts. Photosynth Res 24: 209–220
Thielen APGM and VanGorkom HJ (1981) Quantum efficiency and antenna size of photosystem II, II and I in tobacco chloroplasts. Biochim Biophys Acta 635: 111–120
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hsu, BD., Lee, JY. Characterization of the photosystem II centers inactive in plastoquinone reduction by fluorescence induction. Photosynth Res 27, 143–150 (1991). https://doi.org/10.1007/BF00033253
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00033253