Abstract
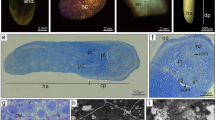
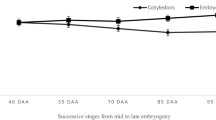
Desiccation tolerance of broccoli microspore-derived embryos was induced by exogenous application of abscisic acid (ABA). Embryos, which were desiccated to about 10% water content, were estimated for viability after rehydration. Survival was dependent on the ABA concentration and the development stage of embryo, but not on the length of exposure period to ABA or genotype. Cotyledonary stage embryos acquired the highest desiccation tolerance when treated with 1×10-4M ABA. Under this condition, on average 27–48% of the desiccated embryos could convert into plants. Embryos treated with 1×10-6M ABA or no ABA or earlier development-staged embryos, such as globular and heart stages, lost viability after desiccation. A one day exposure to ABA had the similar effect on the induction of desiccation tolerance as a 7-day treatment. The dried embryos maintained their ability of plant conversion after three months of storage under room conditions. The plants derived from the desiccated embryos were not different in the morphology or ploidy level from those from non-desiccated ones.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- RH:
-
relative humidity
References
Anandarajah K, Kitto L, Beversdorf WD & McKersie BD (1991) Induction of desiccation tolerance in microspore-derived embryos of Brassica napus L. by thermal stress. Plant Sci. 77: 119–123
Baker JC, Steel C & Dure LIII (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol. Biol. 11: 277–291
Bartels D, Singh D & Salamini F (1988) Onset of desiccation tolerance during development of the barley embryos. Planta 175: 485–492
Bewley JD & Black M (1982) Physiology and biochemistry of seeds. Springer-Verlag, New York
Bray EA & Beachy RN (1985) Regulation by ABA of β-conglycinin in cultured developing cotyledons. Plant Physiol. 79: 746–750
Brown DCW, Singh J, Takahata Y & Watson E (1990) Artificial seeds: Induction of desiccation tolerance in canola microspore-derived embryos. Abstracts VIIth International Congress on Plant Tissue and Cell Culture: 184
Crouch ML, Tenbarge K, Simon A, Finkelstein R, Scofield S & Solberg L (1985) Storage protein mRNA levels can be regulated by abscisic acid in Brassica embryos. In: van Vloten-Doting L, Groot GSP & Hall TC (Eds) Molecular Form and Function of Plant Genome (pp 555–566). Plenum Press, New York
Datta SK & Potrykus I (1989) Artificial seeds in barley: encapsulation of microspore-derived embryos. Theor. Appl. Genet. 77: 820–824
Dure LIII, Crouch M, Harada J, Ho THD, Mundy J, Quatrano R, Thomas T & Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 12: 475–486
Finkelstein RR, Tenbarge KM, Shumway JE & Crouch ML (1985) Role of ABA in maturation of rapeseed embryos. Plant Physiol. 78: 630–636
Fujii JA, Slade DT, Redenbaugh K & Walker KA (1987) Artificial seeds for plant propagation. TIBTECH 5: 335–339
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
Gray DJ (1989) Effects of dehydration and exogenous growth regulators on dormancy, quiescence and germination of grape somatic embryos. In Vitro Cell Dev. Biol. 25: 1173–1178
Gray DJ, Conger BV & Songstad DD (1987) Desiccation quiescent somatic embryos of orchardgrass for use as synthetic seed. In Vitro Cell Dev. Biol. 23: 29–33
Harada J, DeLisle A, Baden C & Crouch M (1989) Unusual sequence of an abscisic acid-inducible mRNA which accumulates late in Brassica napus development. Plant Mol. Biol. 12: 395–401
Hong B, Uknes SJ & Ho THD (1988) Cloning and characterization of a cDNA encoding a mRNA rapidly induced by ABA in barley aleurone layers. Plant Mol. Biol. 11: 495–506
Kim YH & Janick J (1989) ABA and Polyox-encapsulation on high humidity increased survival of desiccation somatic embryos of celery. HortScience 24: 674–676
Kim YH & Janick J (1991) Abscisic acid and proline improve desiccation tolerance and increase fatty acid content of celery somatic embryos. Plant Cell Tiss. Org. Cult. 24: 83–89
Kitto SL & Janick J (1985) Production of synthetic seeds by encapsulation asexual embryos of carrot. J. Amer. Soci. Hort. Sci. 110: 277–282
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z. Pflanzenphysiol. 105: 427–434
Mundy J & Chua NH (1988) Abscisic acid and water stress induce the expression of a novel rice gene. EMBO J. 7: 2279–2286
Quatrano RS (1986) Regulation of gene expression by abscisic acid during angiosperm embryo development. In: Miflin BJ (Ed) Oxford Surveys of Plant Molecular and Cell Biology 3 (pp 476–477). Oxford Univ Press, Oxford
Redenbaugh K, Paasch BD, Nichol JW, Kossler ME, Viss PR & Walker KA (1986) Somatic seeds: encapsulation of asexual plant embryos. Bio/Technology 4: 797–801
Robichaud CS, Wong J & Sussex IM (1980) Control of in vitro growth of viviparous embryo mutants of maize by abscisic acid. Devlop. Gen. 1: 325–330
Senaratna T, McKersie BD & Bowley SR (1989) Desiccation tolerance of alfalfa (Medicago sativa L.) somatic embryos. Influence of abscisic acid, stress pretreatments and drying rates. Plant Sci. 65: 253–259
Senaratna T, McKersie BD & Bowley SR (1990) Artificial seed of alfalfa (Medicago sativa L.). Induction of desiccation tolerance in somatic embryos. In Vitro Cell Dev. Biol. 26: 85–90
Shimonishi K, Ishikawa M, Suzuki M & Oosawa K (1991) Cryopreservation of melon somatic embryos by desiccation method. Japan. J. Breed. 41: 347–351
Shin DH, Virigool S, Shinozaki KY & Oono K (1991) Survival mechanism of dried calli and regeneration of plants in rice. Jpn. J. Genet. 66: 13–25
Sussex IM & Dale RMK (1979) Hormonal control of storage protein synthesis in Phaseolus vulgaris. In: Rubenstein I, Phillips RL & Green CE (Eds) The Plant Seed: Development, Preservation and Germination (pp 129–141). Academic Press, New York
Takahata Y & Keller WA (1991) High frequency embryogenesis and plant regeneration in isolated microspore culture of Brassica oleracea L. Plant Sci. 74: 235–242
Takahara Y, Brown DCW & Keller WA (1991) Effect of donor plant age and infloresence age on microspore culture of Brassica napus L. Euphytica 58: 51–55
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahata, Y., Brown, D.C.W., Keller, W.A. et al. Dry artificial seeds and desiccation tolerance induction in microspore-derived embryos of broccoli. Plant Cell Tiss Organ Cult 35, 121–129 (1993). https://doi.org/10.1007/BF00032961
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00032961