Abstract
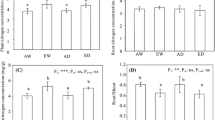
Elevated CO2 (ambient + 35 Pa) increased shoot dry mass production in Avena fatua by ∼ 68% at maturity. This increase in shoot biomass was paralleled by an 81% increase in average net CO2 uptake (A) per unit of leaf area and a 65% increase in average A at the ‘ecosystem’ level per unit of ground area. Elevated CO2 also increased ‘ecosystem’ A per unit of biomass. However, the products of total leaf area and light-saturated leaf A divided by the ground surface area over time appeared to lie on a single response curve for both CO2 treatments. The approximate slope of the response suggests that the integrated light saturated capacity for leaf photosynthesis is ∼ 10-fold greater than the ‘ecosystem’ rate. ‘Ecosystem’ respiration (night) per unit of ground area, which includes soil and plant respiration, ranged from-20 (at day 19) to-18 (at day 40) μmol m-2 s-1 for both elevated and ambient CO2 Avena. ‘Ecosystem’ below-ground respiration at the time of seedling emergence was ∼-10 μmol m-2 s-1, while that occuring after shoot removal at the termination of the experiment ranged from -5 to-6 μmol m-2 s-1. Hence, no significant differences between elevated and ambient CO2 treatments were found in any respiration measure on a ground area basis, though ‘ecosystem’ respiration on a shoot biomass basis was clearly reduced by elevated CO2. Significant differences existed between leaf and ‘ecosystem’ water flux. In general, leaf transpiration (E) decreased over the course of the experiment, possibly in response to leaf aging, while ‘ecosystem’ rates of evapotranspiration (ET) remained constant, probably because falling leaf rates were offset by an increasing total leaf biomass. Transpiration was lower in plants grown at elevated CO2, though variation was high because of variability in leaf age and ambient light conditions and differences were not significant. In contrast, ‘ecosystem’ evapotranspiration (ET) was significantly decreased by elevated CO2 on 5 out of 8 measurement dates. Photosynthetic water use efficiencies (A/E at the leaf level, A/ET at the ‘ecosystem’ level) were increased by elevated CO2. Increases were due to both increased A at leaf and ‘ecosystem’ level and decreased leaf E and ‘ecosystem’ ET.
Similar content being viewed by others
References
Bazzaz FA (1990) The response of natural ecosystem's to the rising global CO2 levels. Ann Rev Ecol Syst 21: 167–196
Björkman O, Berry J, Mooney HA, Nicholson F and Catanzaro B (1972) Physiological adaptation to diverse environments: Approaches and facilities to study plant responses to contrasting thermal and water regimes. CIW Yearbook 72: 393–403
Bloom AJ, Chapin FSIII and Mooney HA (1985) Resource limitation in plants-an economic analogy. Ann Rev Ecol Syst 16: 363–392
Bunce JA (1992) Light, temperature and nutrients as factors in photosynhtetic adjustment to an elevated concentration of carbon dioxide. Physiol Plant 86: 173–179
Chazdon RL and Field CB (1987) Photographic estimation of photosynthetically active radiation: Evaluation of a computerized technique. Oecologia 73: 525–532
Conway TJ, Tans PP, Waterman LS, Thoning KW, Kitzis DR, Masarie KA and Zhang N (1994) Evidence for interannual variability of the carbon cycle from the National Oceanic and Atmospheric Administration/Climate Monitoring and Diagnostics Laboratory Global Air Sampling Network. J Geophys Res 99(D11): 22,831–22,855
Drake BG (1989) Effects of elevated carbon dioxide on Chesapeake Bay Wetlands. V. Ecosystem and whole plant responses. Response of Vegetation to carbon dioxide. US Dept of Energy, Washington, DC, pp 105
Eamus D (1991) The interaction of rising CO2 and temperatures with water use efficiency. Plant Cell Environ 14: 843–852
Field CB, Chapin FSIII, Matson PA and Mooney HA (1992) Responses of terrestrial ecosystem's to the changing atmosphere: A resource-based approach. Ann Rev Ecol Syst 23: 201–235
Field CB, Chapin PS III, Chiariello NK, Holland EA and Mooney HA (1995) The Jasper Ridge CO2 experiment: Design and motivation. In H Mooney & GW Koch (eds) Ecosystem Responses to Elevated Atmospheric CO2. Academic Press (in press)
Fredeen AL, Koch GW and Field CB (1995a) Effects of atmospheric CO2 enrichment on ‘ecosystem’ CO2 exchange in a nutrient and water limited grassland. J Biogeogr 22 (in press)
Fredeen AL, Randerson JT, Holbrook NM and Field CB (1995b) Elevated atmospheric CO2 increases late-season water availability in a water-limited grassland ‘ecosystem’. Plant Cell Environment (submitted)
Garbutt K, Williams WE and Bazzaz FA (1990) Analysis of the differential response of five annuals to elevated CO2 during growth. Ecology 71(3): 1185–1194
Houghton JT, Jenkins GJ and Ephraums JJ (1990) Climate Change: The IPCC Scientific Assessment, p 365. Cambridge University Press. Cambridge
Idso SB and Kimball BA (1993) Tree growth in carbon dioxide enriched air and its implications for global carbon cycling and maximum levels of atmospheric CO2. Global Biogeochem Cycles 7(3): 537–555
Jackson RB, Sala OE, Field CB and Mooney HA (1994) CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 98: 257–262
Kimball BA (1983) Carbon dioxide and agricultural yield: An assemblage and analysis of 430 prior observations. Agron J 75: 779–788
Luo Y, Jackson RB, Field CB and Mooney HA (1995) Increased soil respiration with elevated CO2. (submitted)
Mooney HA, Drake BG, Luxmoore RJ, Oechel WC and Pitelka LF (1991) Predicting ecosystem responses to elevated CO2 concentrations. Bioscience 41(2): 96–104
Morison JIL (1985) Sensitivity of stomata and water use efficiency to high CO2. Plant Cell Environ 8: 467–474
Natr L (1972) Influence of mineral nutrients on photosynthesis of higher plants. Photosynthetica 6: 80–99
Natr L (1975) Influence of mineral nutrition on photosynthesis and the use of assimilates. In: Photosynthesis and Productivity in Different Environments, Int Biol Prog, vol 3, pp 537–555. Cambridge Press, Cambridge, UK
Nie D, He H, Mo G, Kirkham MB and Kanemasu ET (1992) Canopy photosynthesis and evapotranspiration of rangeland plants under doubled carbon dioxide in closed-top chambers. Agron For Met 61: 205–217
Norby RJ, Gunderson CA, Wullschleger SD, O'Neill EG and McCracken MK (1992) Productivity and compensatory responses of yellow-poplar trees in elevated CO2. Nature 357: 322–324
Oechel WC and Strain BR (1985) Native species responses to increased atmospheric carbon dioxide concentrations. In: Strain BR & Cure JA (eds) Direct Effects of Increasing Carbon Dioxide on Vegetation, pp 117–154. US Dept of Energy, Washington DC
Oechel WC, Cowles S, Grulke N, Hastings SJ, Lawrence B, Prudhomme T, Riechers G, Strain B, Tissue D and Vourlitis G (1994) Transient nature of CO2 fertilization in Arctic tundra. Nature 371: 500–503
Owensby CE, Coyne PI, Ham JM, Auen JM and Knapp AK (1993) Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2. Ecol Appl 3(4): 644–653
Radoglou KM, Aphalo P and Jarvis PG (1992) Response of photosynthesis, stomatal conductance and water use efficiency to elevated CO2 and nutrient supply in acclimated seedlings of Phaseolus vulgaris L. Ann Bot 70: 257–264
Silvola J and Ahlholm U (1992) Photosynthesis in willows (Salix x dasyclados) grown at different CO2 concentrations and fertilization levels. Oecologia 91: 208–213
Tans PP, Fung IY and Takahashi T (1990) Observational constraints on the global atmospheric CO2 budget. Science 247: 1431–1438
Vitousek PM and Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochem 13: 87–115
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fredeen, A.L., Field, C.B. Contrasting leaf and ‘ecosystem’ CO2 and H2O exchange in Avena fatua monoculture: Growth at ambient and elevated CO2 . Photosynth Res 43, 263–271 (1995). https://doi.org/10.1007/BF00029939
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00029939