Abstract
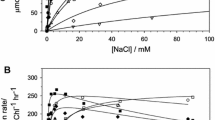
The effect of Cl depletion on the sensitivity of the oxygen-evolving complex of Photosystem II (PS II) to heat treatment was examined by a parallel study of the Hill activity (H2O→2,6-dichlorophenolindophenol), Cl- binding (by 35Cl-NMR) and Mn release (by EPR). The extent of thermal inactivation in spinach thylakoids was found to depend on the degree of Cl- depletion in the sample. In partially Cl--depleted thylakoids, mild heating (38°C, 3 min) was found to eliminate inflections in plots of both Hill activity versus [Cl-] (at low light intensity) and excess 35Cl-NMR linewidth versus [Cl-] (in the dark). In PS II membranes, the same treatment reduced the differences between the linewidth maxima and minima, particularly in the region of 0.3 mM and 7.0 mM Cl-, as compared to unheated membranes. These results indicate that mild heating affects the Cl--binding domains within the oxygen-evolving complex, OEC, EPR measurements of the temperature dependence of Mn release from heated thylakoids show that Mn release begins to correlate with the loss of Hill activity only at higher temperatures, where the OEC is already substantially inactivated. We conclude from these studies that the Cl--binding domains of the OEC constitute a principal site of damage by heat treatment.
Similar content being viewed by others
References
Abramowicz DA and Dismukes GC (1984) Manganese proteins isolated from spinach thylakoid membranes and their role in O2 evolution. II. A binuclear manganese-containing 34 kilodalton protein, a probable component of the water dehydrogenase enzyme. Biochim Biophys Acta 765: 318–328
Baianu IC, Critchley C, Govindjee and Gutowsky HS (1984) NMR study of chloride ion interactions with thylakoid membranes. Proc Natl Acad Sci USA 81: 3713–3717
Blankenship RE and Sauer K (1974) Manganese in photosynthetic oxygen evolution. I. Electron paramagnetic resonance study of the environment of manganese in Tris-washed chloroplasts. Biochim Biophys Acta 347: 252–266
Cheniae GM and Martin IF (1966) Studies on the function of manganese in photosynthesis. Brookhaven Symp Biol 19: 409–417
Chiancone E, Norne JE, Forsen S, Antonini E and Wyman J (1972) Nuclear magnetic resonance quadrupole relaxation studies of chloride binding to human oxy- and deoxyhaemoglobin. J Mol Biol 70: 675–688
Coleman WJ and Govindjee (1987) A model for the mechanism of chloride activation of oxygen evolution in Photosystem II. Photosynthesis Res 13: 199–223
Coleman WJ, Baianu IC, Gutowsky HS and Govindjee (1984) The effect of chloride and other anions on the thermal inactivation of oxygen evolution in spinach thylakoids. In: Sybesma C (ed.) Advances in Photosynthesis Research, Vol. 1, pp. 283–286. The Hague: Martinus Nijhoff/Dr. W. Junk
Coleman WJ, Govindjee and Gutowsky HS (1987a) 35Cl-NMR measurement of chloride binding to the oxygen-evolving complex of spinach photosystem II. Biochim Biophys Acta 894: 443–452
Coleman WJ, Govindjee and Gutowsky HS (1987b) The location of the chloride binding sites in the oxygen-evolving complexes of Photosystem II. Biochim Biophys Acta 894: 453–459.
Cramer WA, Whitmarsh J and Low PS (1981) Differential scanning calorimetry of chloroplast membranes: identification of an endothermic transition associated with the water-splitting complex of photosystem II. Biochemistry 20: 157–162
Critchley C, Baianu IC, Govindjee and Gutowsky HS (1982) The role of chloride in O2 evolution by thylakoids from salt-tolerant higher plants. Biochim Biophys Acta 682: 436–445
Critchley C and Sargeson AM (1984) A manganese-chloride cluster as the functional centre of the O2 evolving enzyme in photosynthetic systems. FEBS Lett 177: 2–5
Dismukes GC (1986) The metal centers of the photosynthetic oxygen-evolving complex. Photochem Photobiol 43: 99–115
Fukada H, Sturtevant JM and Quiocho FA (1983) Thermodynamics of the binding of L-arabinose and of D-galactose to the L-arabinose-binding protein of Escherichia coli. J Biol Chem 258: 13193–13198
Gerhart JC and Pardee AB (1962) The enzymology of controlled-feedback inhibition. J Biol Chem 237: 891–896
Gerhart JC and Pardee AB (1963) The effect of the feedback inhibitor, CTP, on the subunit interactions in aspartate transcarbamylase. Cold Spring Harbor Symp. Quant Biol 28: 491–496
Hind G, Nakatani HY and Izawa S (1969) The role of Cl- in photosynthesis. I. the Cl- requirement of electron transport. Biochim Biophys Acta 172: 277–289
Hinz H-J (1986) Thermodynamic parameters for protein-protein and protein-ligand interation by differential scanning microcalorimetry. Methods Enzymol 130: 59–79
Homann PH (1987) The relations between the chloride, calcium and polypeptide requirements of photosynthetic water oxidation. J Bioenerg Biomembr 19: 105–123
Homann PH, Johnson JD and Pfister VR (1983) Interaction of protons with Photosystem II. In: Inoue Y, Crofts AR, Govindjee, Murata N, Renger G and Satoh K (eds) The Oxygen Evolving System of Photosynthesis, pp 283–292. Tokyo, Orlando and San Diego: Academic Press
Izawa S, Muallem A and Ramaswamy NK (1983) Chloride ion-sensitive inactivation of oxygen evolving centers. In: Inoue Y, Crofts AR, Govindjee, Murata N, Renger G and Satoh K (eds) The Oxygen Evolving System of Photosynthesis, pp. 293–302. Tokyo, Orlando and San Diego: Academic Press
Katoh S and San Pietro A (1967) Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys 122: 144–152
Khanna R, Rajan S, Govindjee and Gutowsky HS (1983) Effects of physical and chemical treatments on chloroplast manganese. NMR and ESR studies. Biochim Biophys Acta 725: 10–18
Kimimura M and Katoh S (1972) On the functional site of manganese in photosynthetic electron transport system. Plant and Cell Physiol 13: 287–296
Krishnan M and Mohanty P (1984) Reactivation by chloride of Hill activity in heat- and tris-treated thylakoid membranes from Beta vulgaris. Photosynth Res 5: 185–198
Kurganov BI (1982) Allosteric Enzymes. New York: Wiley
Lee JC and Timasheff SN (1981) The stabilization of proteins by sucrose. J Biol Chem 256: 7193–7201
Lee LMY, Krupka RM and Cook RA (1973) Cooperativity in human erythrocyte phospho-fructokinase. Biochemistry 12: 3503–3508
Lozier R, Baginsky M and Butler WL (1971) Inhibition of electron transport in chloroplasts by chaotropic agents and the use of manganese as an electron donor to photosystem II. Photochem Photobiol 14: 323–328
Mansfield R and Barber J (1982) Manganese levels associated with inside-out thylakoid membranes in relation to oxygen evolution. FEBS Lett 140: 165–168
Miller M and Cox RP (1982) Rapid equilibration of added Mn2+ across the chloroplast thylakoid membrane. Photobiochem Photobiophys 4: 243–248
Miller M and Cox RP (1984) Manganese release following inhibition of photosynthetic oxygen evolution by heat treatment: Topology and stoichiometry. In: Sybesma C (ed.) Advances in Photosynthesis Research, Vol. 1. pp 355–358. The Hague: Martinus Nijhoff/ Dr. W. Junk
Nash D, Miyao M and Murata N (1985) Heat inactivation of oxygen evolution in photosystem II particles and its acceleration by chloride depletion and exogenous manganese. Biochim Biophys Acta 807: 127–133
Privalov PL (1982) Stability of Proteins. Adv Protein Chem 35: 1–104
Sandusky PO and Yocum CF (1983) The mechanism of amine inhibition of the photosynthetic oxygen evolving complex. FEBS Lett 162: 339–343
Stadtman ER (1966) Allosteric regulation of enzyme activity. In: Nord FF (ed) Advances in Enzymology, Vol. 28, pp. 41–154. New York: Interscience
Stadtman ER (1970) Mechanisms of enzyme regulation in metabolism. In: Boyer PD (ed.) The Enzymes, 3rd ed., Vol. 1, pp., 397–459. New York: Academic Press
Velthuys B (1983) Spectrophotometric methods of probing the donor side of Photosystem II. In: Inoue Y, Crofts AR, Govindjee, Murata N, Renger G and Satoh K (eds) The Oxygen Evolving System of Photosynthesis, pp. 83–90. Tokyo, Orlando and San Diego: Academic Press
Webster RE and Gross SR (1965) The α-isopropylmalate synthetase of Neurospora. I. The kinetics and end product control of α-isopropylmalate synthetase function. Biochemistry 4: 2309–2318
Weis E (1982) The influence of metal cations and pH on the heat sensitivity of photosynthetic oxygen evolution and chlorophyll fluorescence in spinach chloroplasts. Planta 154: 41–47
Wydrzynski TJ (1982) Oxygen evolution in photosynthesis. In: Govindjee (ed.) Photosynthesis: Energy conversion by plants and bacteria, vol. 1, pp. 469–506. New York: Academic Press
Wydrzynski TJ and Sauer K (1980) Periodic changes in the oxidation state of manganese in photosynthetic oxygen evolution upon illumination with flashes. Biochim Biophys Acta 589: 56–70
Yachandra VK, Guiles RD, McDermott A, Britt RD, Drexheimer SL, Sauer K and Klein MP (1986) The state of manganese in the photosynthetic apparatus. 4. Structure of the manganese complex in Photosystem II studied using EXAFS spectroscopy. The S1 state of the O2-evolving complex from spinach. Biochim Biophys Acta 850: 324–332
Yamashita S and Butler WL (1968) Inhibition of chloroplasts by UV-irradiation and heat-treatment. Plant Physiol 43: 2037–2040
Yacum CF, Yerkes CT, Blankenship RE, Sharp RR and Babcock GT (1981) Stoichiometry, inhibitor sensitivity, and organization of manganese associated with photosynthetic oxygen evolution. Proc Natl Acad Sci USA 78: 7507–7511
Zyk N, Citri N and Moyed HS (1969) Conformative response of xanthosine 5′-phosphate aminase. Biochemistry 8: 2787–2794
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coleman, W.J., Govindjee & Gutowsky, H.S. The effect of chloride on the thermal inactivation of oxygen evolution. Photosynth Res 16, 261–276 (1988). https://doi.org/10.1007/BF00028844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00028844