Abstract
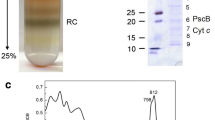
Photosynthetic reaction centers isolated from Heliobacillus mobilis exhibit a single major protein on SDS-PAGE of 47 000 Mr. Attempts to sequence the reaction center polypeptide indicated that the N-terminus is blocked. After enzymatic and chemical cleavage, four peptide fragments were sequenced from the Heliobacillus mobilis apoprotein. Only one of these sequences showed significant specific similarity to any of the protein and deduced protein sequences in the GenBank data base. This fragment is identical with 56% of the residues, including both cysteines, found in the highly conserved region that is proposed to bind iron-sulfur center FX in the Photosystem I reaction center peptide that is the psaB gene product. The similarity to the psaA gene product in this region is 48%.
Redox titrations of laser-flash-induced photobleaching with millisecond decay kinetics on isolated reaction centers from Heliobacterium gestii indicate a midpoint potential of −414 mV with n=2 titration behavior. In membranes, the behavior is intermediate between n=1 and n=2, and the apparent midpoint potential is −444 mV. This is compared to the behavior in Photosystem I, where the intermediate electron acceptor A1, thought to be a phylloquinone molecule, has been proposed to undergo a double reduction at low redox potentials in the presence of viologen redox mediators.
These results strongly suggest that the acceptor side electron transfer system in reaction centers from heliobacteria is indeed analogous to that found in Photosystem I. The sequence similarities indicate that the divergence of the heliobacteria from the Photosystem I line occurred before the gene duplication and subsequent divergence that lead to the heterodimeric protein core of the Photosystem I reaction center.
Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- %C:
-
percent bisacrylamide as a percentage of total acrylamide
- DTT:
-
dithiothreitol
- EPR:
-
electron paramagnetic resonance
- Fe-S:
-
iron-sulfur center
- H. :
-
Heliobacterium
- Hb. :
-
Heliobacillus
- k:
-
one thousand
- Mr :
-
molecular retention
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- RCs:
-
reaction centers
- SDS:
-
sodium dodecyl sulfate
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide electrophoresis
- %T:
-
percent total acrylamide
- Tris:
-
tris(hydroxymethyl)aminomethane
References
Amesz J (1991) Green photosynthetic bacteria and heliobacteria. In: Shively JM and Barton LL (eds) Variations in Autotrophic Life, pp 99–119. Academic Press, London
Barber J (1988) Similarities and differences between Photosystem II and the purple bacterial reaction centers. In: StevensS E Jr and Bryant DA (eds) Light-Energy Transduction in Photosynthesis: Higher Plant and Bacterial Models, pp 178–196. The American Society of Plant Physiologists, Rockville MD
Barry BA, Bender CJ, McIntosh L, Ferguson-Miller S and Babcock GT (1988) Photoaccumulation in Photosystem I does not produce a phylloquinone radical. Israel J Chem 28: 129–132
Beanland TJ (1990) Evolutionary relationships between ‘Qtype’ photosynthetic reaction centres: Hypothesis testing using parsimony. J Theor Biol 145: 535–545
Beck H, Hegeman GD and Whilte D (1990) Fatty acid and lipopolysaccharide analyses of three Heliobacterium spp. FEMS Microbiol Lett 69: 229–232
Beer-Romero P, Favinger JL and Gest H (1988) Distinctive properties of bacilliform photosynthetic heliobacteria. FEMS Microbiol Lett 49: 451–454
Biggins J (1990) Evaluation of selected benzoquinones, naphthoquinones, and anthraquinones as replacements for phylloquinone in the A1 acceptor site of the Photosystem I reaction center. Biochemistry 29: 7259–7264
Biggins J and Mathis P (1988) Functional role of vitamin K1 in Photosystem I of the cyanobacterium Synechocystis 6803. Biochemistry 27: 1494–1500
Blankenship RE (1992) Origin and early evolution of photosynthesis. In: Harman H (ed) Photosynthesis and the Origin of Life, Burlingame. Neil Patterson Publishers, NC in press
Bottin H and Sétif P (1991) Inhibition of electron transfer from A0 to A1 in Photosystem I after treatment in darkness at low redox potential. Biochim Biophys Acta 1075: 331–336
Brettel K (1990) Electron transfer in Photosystem I from Synechococeus sp. under reducing conditions. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 2, pp 627–630. Kluwer, Dordrecht
Brockmann H jr and Lipinski A (1983) Bacteriochlorophyll g. A new bacteriochlorophyll from Heliobacterium chlorum. Arch Microbiol 136: 17–19
Brok M, Vasmel H, Horikx JTG and Hoff AJ (1986) Electron transport components of Heliobacterium chlorum investigated by EPR spectroscopy at 9 and 35 GHz. FEBS Lett 19: 322–326
Bruce BD and Malkin R (1988) Subunit stoichiometry of the chloroplast Photosystem I complex. J Biol Chem 263: 7302–7308
Deinum G, Kramer H, Aartsma TJ, Kleinherenbrink FAM and Amesz J (1991) Fluorescence quenching in Heliobacterium chlorum by reaction centers in the charge separated state. Biochim Biophys Acta 1058: 339–344
Devereux J, Haeberli P and Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res 12: 387–395
Fischer MR (1990) Photosynthetic electron transfer in Heliobacterium chlorum studied by EPR spectroscopy. Biochim Biophys Acta 1015: 471–481
Gest H and Favinger JL (1983) Heliobacterium chlorum, an anoxygenic brownish-green photosynthetic bacterium containing a ‘new’ form of bacteriochlorophyll. Arch Microbiol 136: 11–16
Golbeck JH and Bryant DA (1991) Photosystem I. In: Lee CP (ed) Current Topics in Bioenergetics, Vol. 16, pp 83–177. Academic Press, New York
Golbeck JH and Cornelius JM (1986) Photosystem I charge separation in the absence of centers A and B. I. Optical characterization of center A2 and evidence for its association with a 64-kDa peptide. Biochim Biophys Acta 849: 16–24
Goodhew CF, Brown KR and Pettigrew GW (1986) Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta 852: 288–294
Hauska G (1988) Phylloquinone in Photosystem I: Are quinones the secondary electron acceptors in all types of photosynthetic reaction centers? Trends Biochem Sci 13: 415–416
Hiraishi A (1989) Occurrence of menaquinone as the sole isoprenoid quinone in the photosynthetic bacterium Heliobacterium chlorum. Arch Microbiol 151: 378–379
Hurt EC and Hauska G (1984) Purification of membranebound cytochromes and a photoactive P840 protein complex of the green sulfur bacterium Chlorobium limicola f thiosulfatophilum. FEBS Lett 168: 149–154
Itoh S, Iwaki M and Ikegami I (1987) Extraction of vitamin K-1 from Photosystem I particles by treatment with diethyl ether and is effects on the A1-EPR signal and system I photochemistry. Biochim Biophys Acta 893: 508–516
Iwaki M and Itoh S (1990) Function of substituted quinones as the electron acceptor A1-(phylloquinone) in Photosystem I reaction center. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 2, pp 647–650. Kluwer, Dordrecht
Kjaer B, Scheller HV, Andersen B, Okkels JS and Møller BL (1991) Isolation of the photosynthetic reaction center from Chlorobium limicola., VII International Symposium on Photosynthetic Prokaryotes. Amherst, MA, USA
Knaff DB and Malkin R (1976) Iron-sulfur proteins of the green photosynthetic bacterium Chlorobium. Biochim Acta 430: 244–252
Kobayashi M, Watanabe T, Ikegami I, van de Meent EJ and Amesz J (1991a) Enrichment of bacteriochlorophyll g' in membranes of Heliobacterium chlorum by ether extraction; unequivocal evidence for its existence in vivo. FEBS Lett 284: 129–131
Kobayasjhi M, van de Meent EJ Erkelens C, AmeszJ, Ikegami I and Watanabe T (1991b) Bacteriochlorophyll g epimer as a possible reaction center component of heliobacteria. Biochim Biophys Acta 1057: 89–96
Kück U, Choquet Y, Schneider M, Dron M and Bennoun P (1987) Structure and transcriptional analysis of two homologous genes for the P700 chlorophyll a-apoprotein in Chlamydomonas reinhardtii: Evidence for in vivo trans splicing. EMBO J 6: 2185–2195
Madigan MT (1992) The family Heliobacteriaceae. In: Balows A, Trüper HG, Dworkin M, Schleifer KH and Harder W (eds) The Prokaryotes 2nd edition: A Handbook on the Biology of Bacteria; Ecophysiology, Isolation, Identification and Applications, Vol II, Chap 90. Springer-Verlag, 1982–1992
Macino LJ, Dean DP and Blankenship RE (1984) Kinetics and thermodynamics of the P870+QA -→P870+QB --reaction in isolated reactions centers from the photosynthetic bacterium Rhodopseudomonas sphaeroides. Biochim Biophys Acta 764: 46–54
Michalski TJ, Hunt JE, Bowman MK, Smith U, Bardeen K, Gest H, Norris JR and Katz JJ (1987) Bacteriopheophytin g: Properties and some speculations on a possible primary role for bacteriochlorophylls b and g in the biosynthesis of chlorophylls. Proc Natl Acad Sci USA 84: 2570–2574
Michel H and Deisenhofer J (1988) Relevance of the photosynthetic reaction center from the purple bacteria to the structure of Photosystem II. Biochemistry 27: 1–7
Nitschke W and Rutherford AW (1991) Photosynthetic reaction centres: variations on a common structural theme? Trends Biochem Sci 16: 241–245
Nitschke W, Feiler U, Lockau W and Hauska G (1987) The photosystem of the green sulfur bacterium Chlorobium limicola contains two early electron acceptors similar to Photosystem I. FEBS Lett 218: 283–286
Nitschke W, Feiler U and Rutherford AW (1990a) Photosynthetic reaction center of green sulfur bacteria studied by EPR. Biochemistry 29: 3834–3842
Nitschke W, Sétif P, Liebl U, Feiler U and Rutherford AW (1990b) Reaction center photochemistry of Heliobacterium chlorum. Biochemistry 29: 11078–11088
Nuijs AM, Van Dorssen RJ, Duysens LNM and Amesz J (1985) Excited states and primary photochemical reactions in the photosynthetic bacterium Heliobacterium chlorum. Proc Natl Acad Sci USA 82: 6965–6968
Prince RC, Gest H and Blankenship RE (1985) Thermodynamic properties of the photochemical reaction center of Heliobacterium chlorum. Biochim Biophys Acta 810: 377–384
Prince RC, Linkletter SJG and Dutton PL (1981) The thermodynamic properties of some commonly used oxidation-reduction mediators, inhibitors and dyes, as determined by polarography. Biochim Biophys Acta 635: 132–148
Rustandi RR, Snyder SW, Feezel L.L, Michalski TJ, Norris JR, Thurnauer MC and Biggins J (1990) Contribution of vitamin K1 to the electron spin polarization in spinach Photosystem I. Biochemistry 29: 8030–8032
Rutherford AW (1988) Photosystem II, the oxygen evolving photosystem. In: Stevens SE Jr and Bryant DA (eds) Light-Energy Transduction in Photosynthesis: Higher Plant and Bacterial Models, pp 163–177. The American Society of Plant Physiologists, Rockville MD
Schägger H and von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379
Sétif P and Bottin H (1989) Identification of electron-transfer reactions involving the acceptor A1 of Photosystem I at room temperature. Biochemistry 28: 2689–2697
Sétif P, Bottin H and Brettel K (1990) Electron transfer reactions of Photosystem I involving the secondary acceptor A1. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 2, pp 539–546. Kluwer, Dordrecht
Smit HWJ, Amesz J and van der Hoeven MFR (1987) Electron transport and triplet formation in membranes of the photosynthetic bacterium Heliobacterium chlorum. Biochim Biophys Acta 893: 232–240
Trost JT and Blankenship RE (1989) Isolation of a photoactive photosynthetic reaction center-core antenna complex from Heliobacillus mobilis. Biochemistry 28: 9898–9904
Van de Meent EJ, Kleinherenbrink FAM and Amesz J (1990) Purification and properties of an antenna-reaction center complex from heliobacteria. Biochim Biophys Acta 1015: 223–230
Van de Meent EJ, Kobayashi M, Erkelens C, van Veelen PA, Amesz J and Watanabe T (1991) Identification of 81-hydroxychlorophyll a as a functional reaction center pigment in heliobacteria. Biochim Biophys Acta 1058: 356–362
Watanabe T and Kobayashi M (1990) Quantitation of reaction centers by HPLC analysis of minor but key chlorophyll-type pigments. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 2, pp 109–112. Kluwer, Dordrecht
Woese CR, Debrunner-Vossbrinck BA, Oyaizu H, Stackebrandt and Ludwig W (1985) Gram positive bacteria: Possible photosynthetic ancestry. Science 229: 762–765
Ziegler K, Lockau W and Nitschke W (1987) Bound electron acceptors of Photosystem I. FEBS Lett 217: 16–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trost, J.T., Brune, D.C. & Blankenship, R.E. Protein sequences and redox titrations indicate that the electron acceptors in reaction centers from heliobacteria are similar to Photosystem I. Photosynth Res 32, 11–22 (1992). https://doi.org/10.1007/BF00028794
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00028794