Abstract
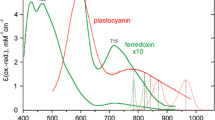
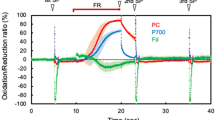
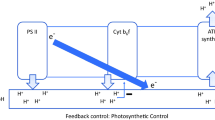
The question of plastoquinone (PQ) concentration and its stoichiometry to photosystem I (PSI) and PSII in spinach chloroplasts is addressed here. The results from three different experimental approaches were compared. (a) Quantitation from the light-induced absorbance change at 263 nm (ΔA263) yielded the following ratios (mol:mol); Chl:PQ=70:1, PQ:PSI=9:1 and PQ:PSIIα=7:1. The kinetics of PQ photoreduction were a monophasic but non-exponential function of time. The deviation of the semilogarithmic plots from linearity reflects the cooperativity of several electron transport chains at the PQ pool level. (b) Estimates from the area over the fluorescence induction curve (Afl) tend to exaggerate the PQ pool size because of electron transfer via PSI to molecular oxygen (Mehler reaction) resulting in the apparent increase of the pool of electron acceptors. The reliability of the Afl method is increased substantially upon plastocyanin inhibition by KCN. (c) Quantitation of the number of electrons removed from PQH2 by PSI, either under far-red excitation or after the addition of DCMU to preilluminated chloroplasts, is complicated due to the competitive loss of electrons from PQH2 to molecular oxygen. The latter is biphasic reaction occurring with half-times of about 2 s (30–40% of PQH2) and of about 60 s (60–70% of PQH2).
Similar content being viewed by others
Abbreviations
- Afl :
-
area over the fluorescence induction curve
- Chl:
-
chlorophyll
- Cyt:
-
cytochrome
- DCMU:
-
3-(3′,4′-dichlorophenyl)-1,1-dimethylurea
- PQ:
-
plastoquinone
- PS:
-
photosystem
- P700:
-
reaction center of PSI
- Q:
-
primary quinone acceptor of PSII
- Tricine:
-
N-tris (hydroxymethyl) methyl glycine
- Triton X-100:
-
octyl phenoxy polyethoxyethanol
References
Amesz J (1973) The function of plastoquinone in photosynthetic electron transport. Biochim Biophys Acta 301:35–51
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Berthold DA, Babcock GT and Yocum CF (1981) A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. EPR and electron-transport properties. FEBS Lett 134:231–234
Duysens LNM and Sweers HE (1963) Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In Studies on microalgae and photosynthetic bacteria, pp. 353–372. Tokyo: University of Tokyo Press
Ghirardi ML and Melis A (1983) Localization of photosynthetic electron transport components in mesophyll and bundle-sheath chloroplasts of Zea mays. Arch Biochem Biophys 202:330–341
Gimmler H (1977) Photophosphorylation in vivo. In Trebst A and Avron M, eds. Encyclopedia of plant physiology 5, pp. 448–472. Berlin: Springer-Verlag
Glick RE, McCauley SW and Melis A (1985) Effect of light quality on chloroplast membrane organization and function. Planta (in press)
Graan T and Ort DR (1984) Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem 259:14003–14010
Haehnel W (1984) Photosynthetic electron transport in higher plants. Ann Rev Plant Physiol 35:659–693
Hauska G (1977) Plasto- and ubiquinone as translocators of electrons and protons through membranes. A facilitating role of the isoprenoid side chain. FEBS Lett 79:345–347
Henninger MD and Crane FL (1966) Electron transport in chloroplasts. I. A combined requirement for plastoquinones A and C for photoreduction of 2,6-dichloroindophenol. J Biol Chem 241:5190–5196
Hiyama T and Ke B (1972) Difference spectra and extinction coefficients of P700. Biochim Biophys Acta 267:160–171
Kyle DJ and Zalik S (1982) Photosystem II activity, plastoquinone A levels, and fluorescence characterization of a virescens mutant of barley. Plant Physiol 70: 1026–1031
Lam E, Baltimore B, Ortiz W, Chollar S, Melis A and Malkin R (1983) Characterization of a resolved oxygen-evolving photosystem II preparation from spinach thylakoids. Biochim Biophys Acta 724:201–211
Lavergne J (1982) Two types of primary acceptors in chloroplasts photosystem II. II. reduction in two successive photoacts. Photobiochem. Photobiophys 3:273–285
Malkin S (1968) Kinetic studies on electron transport components in isolated chloroplasts. I. The effect of the pool of electron carriers between the two photosystems on P700 changes. Biochim Biophys Acta 223:240–250
Malkin S, Kok B (1966) Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta 126:413–432
Marsho TV and Kok B (1970) Interaction between electron transport components in chloroplasts. Biochim Biophys Acta 223:240–250
Meiburg RF, VanGorkom HJ, VanDorssen RJ (1984) Non-electrogenic charge recombination in photosystem II as a source of sub-millisecond luminescence. Biochim Biophys Acta 765:295–300
Melis A (1982) Kinetic analysis of P-700 photoconversion: effect of secondary electron donation and plastocyanin inhibition. Arch Biochim Biophys 217:536–545
Melis A (1984) Light regulation of photosynthetic membrane structure, organization and function. J Cell Biochem 24:271–285
Melis A (1985) Functional properties of photosystem IIβ in spinach chloroplasts. Biochim Biophys Acta (in press)
Melis A and Brown JS (1980) Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosysnthetic membranes. Proc Natl Acad Sci USA 77:4712–4716
Melis A and Hart RW (1980) A laboratory-constructed sensitive difference spectrophotometer for the ultraviolet, visible and far-red region of the spectrum. Carnegie Institution Yearbook 79:170–172
Melis A and Harvey GW (1981) Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim Biophys Acta 637:138–145
Nakatani HY (1983) Inhibition of photosynthetic oxygen evolution in thylakoids by cyanide. Plant Cell Physiol 24:467–472
Ouitrakul R and Izawa S (1973) Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. II. Action-specific inhibition by potassium cyanide. Biochim. Biophys. Acta 305:105–118
Pulles MPJ, VanGorkom HJ and Verschor GAM (1976) Primary reactions of photosystems II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta 440:98–106
Schmidt-Mende P and Rumberg B (1968) Zur Plastochinonreduktion bei der photosynthese. Z Naturforsch 236:225–238
Siggel U, Renger G, Stiel HH and Rumberg B (1972) Evidence for electronic and ionic interaction between electron transport chains in chloroplasts. Biochim Biophys Acta 256:328–335
Stiehl HH and Witt HT (1968) Die Kurzzeitigen ultravioletten differenzspektren bei der photosynthese. Z Naturforsch 23:220–224
Stiehl HH and Witt HT (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z. Naturforsch. 24:1588–1598
Thielen APGM and VanGorkom KH (1981) Redox potentials of electron acceptors in photosystem IIα and IIβ. FEBS Lett 129:205–209
Vernotte C, Etienne AL and Briantais J-M (1979) Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta 545: 519–527
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McCauley, S.W., Melis, A. Quantitation of plastoquinone photoreduction in spinach chloroplasts. Photosynth Res 8, 3–16 (1986). https://doi.org/10.1007/BF00028472
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00028472