Abstract
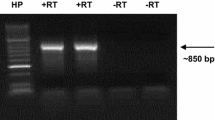
We report the isolation and characterization of seven nuclear genes encoding polyphenol oxidase (PPO) in tomato (Lycopersicon esculentum cv. VFNT Cherry). The seven genes (PPOs A, A′, B, C, D, E and F) fall into three structural classes (I, II, and III) based on Eco RI and Hind III restriction fragment length polymorphisms (RFLP). RFLP mapping and PFGE analysis demonstrated that the genes reside on chromosome 8, and may be clustered within a 165 kb region. Phage insert mapping demonstrated PPO E and PPO F (both class III), and PPOs B, D and A (classes I, II and I respectively) are grouped within separate 12.4 kb clusters. The complete nucleotide sequence was determined for each gene. Comparison to cDNAs revealed that the PPOs lack introns. A transcript of about 2 kb is expected for each PPO. Each PPO possesses a region encoding a transit peptide characteristic of polypeptides targeted to the thylakoid lumen. Predicted precursor polypeptides range in mass from 66 to 71 kDa and predicted mature polypeptides range from 57 to 62 kDa. All the PPOs encode two putative copper-binding sites characteristic of bacterial, fungal and mammalian tyrosinases. Five of the seven PPOs possess divergent DNA sequences in their 5′ promoter regions. These flanking sequence differences may regulate the differential expression of PPO genes.
Similar content being viewed by others
References
Arumuganathan K, Earle ED: Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9: 208–218 (1991).
Benton WD, Davis RW: Screening λgt recombinant clones by hybridization to single plaques in situ. Science 196: 180 (1977).
Bouthyette PY, Eannetta NT, Hannigan KJ, Gregory P: Solanum berthaultii trichomes contain unique polyphenoloxidases and a peroxidase. Phytochemistry 26: 2949–2954 (1987).
Cary JW, Lax AR, Flurkey WH: Cloning and characterization of cDNAs coding for Vicia faba polyphenol oxidase. Plant Mol Biol 20: 245–253 (1992).
Chu G, Vollrath D, Davis RW: Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science 234: 1582–1585 (1986).
Cordes S, Deikman J, Margossian LJ, Fischer RL: Interaction of a developmentally regulated DNA-binding factor with sites flanking two different fruit-ripening genes from tomato. Plant Cell 1: 1025–1034 (1989).
Davis RW, Thomas M, Cameron J, St.John TP, Scherer S, Padgett RA: Rapid DNA isolations for enzymatic and hybridization analysis. Meth Enzymol 65: 404–406 (1980).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res 12: 387–395 (1984).
Duffy SS, Felton G: Enzymatic antinutritive defenses of the tomato plant against insects. In: Hedin P (ed) Naturally Occurring Pest Bioregulators, pp. 167–197. American Chemical Society, Washington DC (1991).
Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction fragments to a high specific activity. Anal Biochem 132: 6–13 (1983).
Felton GW, Donato K, Del Vecchio RJ, Duffey SS: Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. Chem Ecol 15: 2667–2694 (1989).
Flurkey WH: In vitro biosynthesis of Vicia faba polyphenoloxidase. Plant Physiol 79: 564–567 (1985).
Flurkey WH: Polyphenoloxidase in higher plants. Plant Physiol 81: 614–618 (1986).
Ganal MW, Bonierbale MW, Roeder MS, Park WD, Tanksley SD: Genetic and physical mapping of the patatin genes in potato and tomato. Mol Gen Genet 225: 501–509 (1991).
Ganal MW, Tanksley SD: Analysis of tomato DNA by pulsed field gel electrophoresis. Plant Mol Biol Rep 7: 17–27 (1989).
Ganesa C, Fox MT, Flurkey WH: Microheterogeneity in purified broad bean polyphenol oxidase. Plant Physiol 98: 472–479 (1992).
Gil A, Proudfoot NJ: A sequence downstream of AAUAAA is required for rabbit β-globin mRNA 3′-end formation. Nature 312: 473–473 (1984).
Gregory P, Tingey WM, Ave' DA, Bouthyette PY: Potato glandular trichomes: a physiochemical defense mechanism against insects. In: Green MB, Hedin PA (eds) Natural Resistance of Plants to Pests: Roles of Allelochemicals, pp. 160–167. American Chemical Society, Washington DC (1986).
Hunt MD, Eannetta NT, Yu H, Newman SM, Steffens JC: Cloning and expression of potato polyphenol oxidase. Plant Mol Biol 21: 59–68 (1993).
Keegstra K, Olsen LJ, Theg SM: Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol Plant Mol Biol 40: 471–501 (1989).
Kowalski SP: Insect resistance in potato: Purification of a polyphenol oxidase from the type A glandular trichomes of Solanum berthaultii Hawkes. Ph.D. thesis, Cornell University, Ithaca, NY (1989).
Kowalski SP, Eannetta NT, Hirzel AT, Steffens JC: Purification and characterization of polyphenol oxidase from glandular trichomes of Solanum berthaultii. Plant Physiol 100: 677–684 (1992).
Kyte J, Doolittle RF: A simple method for displaying the hydropathic character of a protein. Mol Biol 157: 105–132 (1982).
Lander ES, Green P, Abrahanson J, Barlow A, Daly MJ, Lincoln SE, Newburg L: MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181 (1987).
Lanker T, King TG, Arnold SW, Flurkey WH: Active, inactive and in vitro synthesized forms of polyphenoloxidase during leaf development. Physiol Plant 69: 323–329 (1987).
Lax AR, Vaughn KC: Colocalization of polyphenol oxidase and photosystem II proteins. Plant Physiol 96: 26–31 (1991).
Lerch K: Protein and active-site structure of tyrosinase. In: JT Bagnara (ed) Advances in Pigment Cell Research, pp. 85–98. Alan R. Liss, New York (1988).
Mayer AM: Polyphenol oxidases in plants-recent progress. Phytochemistry 26: 11–20 (1987).
Mayer AM, Harel E: Polyphenol oxidases in plants. Phytochemistry 18: 193–215 (1979).
Mayer AM, Harel E: Phenoloxidases and their significance in fruit and vegetables. In: Fox PF (ed) Food Enzymology, pp. 373–398. Elsevier Science Publishers, New York (1991).
Robinson SP, Dry IB: Broad bean leaf polyphenol oxidase is a 60-kilodalton protein susceptible to proteolytic cleavage. Plant Physiol 99: 317–323 (1992).
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi RG, Horn GT, Mullis KB, Erlich HA: Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491 (1988).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Seed B, Parker RC, Davidson N: Representation of DNA sequences in recombinant DNA Libraries prepared by restriction enzyme partial digestion. Gene 19: 201–209 (1982).
Shahar T, Hennig N, Gutfinger T, Hareven D, Lifschitz E: The tomato 66.3 kD polyphenoloxidase gene: Molecular identification and developmental expression. Plant Cell 4: 135–147 (1992).
Smeekens S, Bauerie C, Hageman J, Keegstra K, Weisbeek P: The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell 46: 365–375 (1986).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. Mol Biol 98: 503–517 (1975).
Steffens JC, Kowalski SP, Yu H: Characterization of glandular trichome and plastid polyphenol oxidases of potatoes. In: Vayda M, Park W (eds) Molecular Biology of the Potato, pp. 103–112. C.A.B. International, Oxon (1990).
Tanaka T, Weisblum B: Construction of a colicin E1-R factor composite plasmid in vitro: Means for amplification of deoxyribonucleic acid. Bacteriology 121: 354–362 (1975).
Tanksley SD, Ganal MW, Prince JP, de Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovanonni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O, Roder MS, Wing RA, Wu W, Young ND: High density molecular linkage maps of the tomato and potato genomes: Biological inferences and practical applications. Genetics 132: 1141–1160 (1992).
Vaughn KC, Duke SO: Tentoxin-induced loss of plastidic polyphenol oxidase. Physiol Plant 53: 421–428 (1981).
Vaughn KC, Duke SO: Tissue localization of polyphenol oxidase in Sorghum. Protoplasma 108: 319–327 (1981).
Vaughn KC, Duke SO: Tentoxin stops the processing of polyphenol oxidase into an active protein. Physiol Plant 60: 257–261 (1984).
Vaughn KC, Lax AR, Duke SO: Polyphenol oxidase: The chloroplast oxidase with no established function. Physiol Plant 72: 659–665 (1988).
Wahl GM, Berger SL: Screening colonies or plaques with radioactive nucleic acid probes. Meth Enzymol 152: 415–421 (1987).
Yu H: Cloning, Characterization and Expression of Tomato Polyphenol Oxidases. Ph.D. thesis. Cornell University, Ithaca, NY (1992).
Yu H, Kowalski SP, Steffens JC: Comparison of polyphenol oxidase expression in glandular trichomes of Solanum and Lycopersicon species. Plant Physiol 100: 1885–1890 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Newman, S.M., Eannetta, N.T., Yu, H. et al. Organisation of the tomato polyphenol oxidase gene family. Plant Mol Biol 21, 1035–1051 (1993). https://doi.org/10.1007/BF00023601
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00023601