Abstract
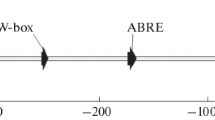
The Arabidopsis FAD7 gene encodes a chloroplast ω-3 fatty acid desaturase that catalyzes the desaturation of lipid-linked dienoic fatty acids (18:2 and 16:2). An 825 bp FAD7 promoter fragment upstream from the transcriptional start point contained several short sequences which were homologous to the cis-elements (box II, G-box, etc.) conserved in many light-responsive genes. We introduced the FAD7 promoter fused to the β-glucuronidase (GUS) or the luciferase (LUC) reporter gene into tobacco plants. The −825 promoter sequence conferred tissue-specific and light-responsive expression to both these reporter genes in transgenic tobacco, indicating that these expressions of the FAD7 gene were regulated mainly at the transcriptional level. Histochemical GUS staining showed that the activity of the FAD7 promoter is restricted to the tissues with chloroplast-containing cells although the staining was noticeably absent in the chloroplast-containing cells associated with vascular systems. The 5′ deletion experiments of the promoter revealed that the −362/ −166 region, containing two putative box II sequences, was responsible for the tissue-specific and light-responsive expression of the FAD7 gene.
Similar content being viewed by others
References
Aoyagi K, Kuhlemeier C, Chua N-H: The pea rbcS-3A enhancer-like element directs cell-specific expression in transgenic tobacco. Mol Gen Genet 213: 179–185 (1988).
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K: Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience, New York (1987).
Bradford MM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Browse J, McCourt P, Somerville C: A mutant of Arabidopsis deficient in C18:3 and C16:3 leaf lipids. Plant Physiol 81: 859–864 (1986).
Browse J, Somerville C: Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42: 467–506 (1991).
Castresana C, Garcia-Luque I, Alonso E, Malik VS, Cashmore AR: Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J 7: 1929–1936 (1988).
Datta N, Cashmore AR: Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell 1: 1069–1077 (1989).
Dean C, Favreau M, Bedbrook J, Dunsmuir P: Sequences 5′ to translation start regulate expression of petunia rbcS genes. Plant Cell 1: 209–215 (1989).
Dehesk K, Bruce WB, Quail PH: A trans-acting factor that binds to a GT-motif in a phytochrome gene promoter. Science 250: 1397–1399 (1990).
Elliot RC, Pedersen TJ, Fristensky B, White MJ, Dickey LF, Thompson WF: Characterization of a single copy gene encoding ferredoxin I from pea. Plant Cell 1: 681–690 (1989).
Elliot RC, Dickey LF, White MJ, Thompson WF: cis-acting elements for light regulation of pea ferredoxin I gene expression are located within transribed sequences. Plant Cell 1: 691–698 (1989).
Flieger K, Tyagi A, Sopory S, Cseplö A, Herrmann RG, Oelmüller R: A 42 bp promoter fragment of the gene for subunit III of photosystem I (psaF) is crucial for its activity. Plant J 4: 9–17 (1993).
Gidoni D, Brosio P, Bond-Nutter D, Bedbrook J, Dunsmuir P: Novel cis-acting elements in petunia Cab gene promoters. Mol Gen Genet 215: 337–344 (1989).
Gilmartin PM, Sarokin L, Memelink J, Chua N-H: Molecular light switches for plant genes. Plant Cell 2: 369–378 (1990).
Green PJ, Yong M-H, Cuozzo M, Kano-Murakami Y, Silverstein P, Chua N-H: Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene. EMBO J 7: 4035–4044 (1988).
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Iba K, Gibson S, Nishiuchi T, Fuse T, Nishimura M, Arondel V, Hughly S, Somerville C: A gene encoding a chloroplast ω-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana. J Biol Chem 268: 24099–24105 (1993).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Kay SA, Keith B, Shinozaki K, Chye M-L, Chua N-H: The rice phytochrome gene: structure, autoregulated expression, and binding of GT-1 to a conserved site in the 5′ upstream region. Plant Cell 1: 351–360 (1989).
Kodama H, Hamada T, Horiguchi G, Nishimura M, Iba K: Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty acid desaturase in transgenic tobacco. Plant Physiol 105: 601–605 (1994).
Lam E, Chua N-H: GT-1 binding site confers light responsive expression in transgenic tobacco. Science 248: 471–474 (1990).
Lemieux B, Miquel M, Somerville C, Browse J: Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet 80: 234–240 (1990).
Manzara T, Gruissem W: Organization and expression of the genes encoding ribulose-1,5-bisphosphate carboxylase in higher plants. Photosynth Res 16: 117–139 (1988).
McConn M, Hugly S, Browse J, Somerville C: A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol 106: 1609–1614 (1994).
McCourt P, Kunst L, Browse J, Somerville CR: The effect of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol 84: 353–360 (1987).
Millar AJ, Short SR, Chua N-H, Kay SA: A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4: 1075–1087 (1992).
Murashige T, Skoog F: A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Murphy DJ, Stumpf PK: Light-dependent induction of polyunsaturated fatty acid biosynthesis in greening cucumber cotyledons. Plant Physiol 63: 328–335 (1979).
Ohnishi J, Yamada M: Glycerolipid synthesis in Avena leaves during greening of etiolated seedlings. IV. Effect of light on fatty acid desaturation. Plant Cell Physiol 24: 1553–1557 (1983).
Ow DW, Wood KV, De Luca M, de Wet JR, Helinski DR, Howell SH: Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234: 856–859 (1986).
Reddy PS, Padayatty JD: Effects of 5′ flanking sequences and changes in the 5′ internal control region on the transcription of rice tRNAGly (GCC) gene. Plant Mol Biol 11: 575–583 (1988).
Reed JW, Nagpal P, Poole DS, Furuya M, Chory J: Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 (1993).
Schindler U, Beckmann H, Cashmore AR: TGA1 and G-box binding factors: two distinct classes of Arabidopsis leucine zipper proteins compete for the G-box-like element TGACGTGG. Plant Cell 4: 1309–1319 (1992).
Schindler U, Terzaghi W, Beckmann H, Kadesch T, Cashmore AR: DNA binding site preferences and transcriptional activation properties of the Arabidopsis transcription factor GBF1. EMBO J 11: 1275–1289 (1992).
Somerville C, Browse J: Plant lipids: metabolism, mutants, and membranes. Science 252: 80–87 (1991).
Somerville C, Ogren WL: Isolation of photorespiration mutants of Arabidopsis. In: Edelman M, Hallik RB, Chua N-H (eds) Methods in Chloroplast Molecular Biology, pp. 129–138. Elsevier, Amsterdam (1981).
Stockhaus J, Eckes P, Rocha-Sosa M, Schell J, Willmitzer L: Analysis of cis-active sequences involved in the leaf-specific expression of a potato gene in transgenic plants. Proc Natl Acad Sci USA 84: 7943–7947 (1987).
Thompson WF, White MJ: Physiological and molecular studies of light-regulated nuclear genes in higher plants. Annu Rev Plant Physiol Plant Mol Biol 42: 423–466 (1991).
Williams ME, Foster R, Chua N-H: Sequences flanking the hexameric G-box core CACGTG affect the specificity of protein binding. Plant Cell 4: 485–496 (1992).
Vorst O, van Dam F, Weisbeek P, Smeekens S: Light-regulated expression of the Arabidopsis thaliana ferredoxin A gene involves both transcriptional and post-transcriptional processes. Plant J 3: 793–803 (1983).
Vorst O, Kock P, Lever A, Weterings B, Weisbeek P, Smeekens S: The promoter of the Arabidopsis thaliana plastocyanin gene contains a far upstream enhancer-like element involved in chloroplast-dependent expression. Plant J 4: 933–945 (1993).
Yadav NS, Wierzbicki A, Aegerter M, Caster CS, Pérez-Grau L, Kinney AJ, Hitz WD, Booth JR, Schweiger B, Stecca KL, Allen SM, Blackwell M, Reiter RS, Carlson TJ, Russel SH, Feldmann KA, Pierce J, Browse J: Cloning of higher plant ω-3 fatty acid desaturases. Plant Physiol 103: 467–476 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishiuchi, T., Nakamura, T., Abe, T. et al. Tissue-specific and light-responsive regulation of the promoter region of the Arabidopsis thaliana chloroplast ω-3 fatty acid desaturase gene (FAD7). Plant Mol Biol 29, 599–609 (1995). https://doi.org/10.1007/BF00020987
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020987