Summary
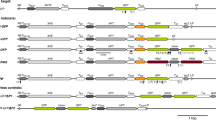
The T-DNA structure and organization in tissues obtained via transformation of tobacco protoplasts with Ti-plasmid DNA was found to be completely different from the T-DNA introduced via Agrobacterium tumefaciens. It is often fragmented. Overlapping copies of T-DNA, having various sizes, as well as separated fragments of T-DNA were detected. The border sequences of 23 basepairs (bp), flanking the T-region in the Ti-plasmid as direct repeats are not used as preferred sequences for integration. Similar results were obtained with a T-region clone lacking one of the TL-borders. This clone, which carried the cytokinin locus and only the right border sequence of TL and the left border sequence of TR, still had the capacity to transform protoplasts. Also the Vir-region of the Ti-plasmid is not required for integration of foreign DNA via DNA transformation. This is demonstrated by the results with the T-region clone mentioned and by the transforming capacity of a Ti-plasmid carrying a mutated Vir-region. Nevertheless, in a number of Ti-plasmid DNA transformants Vir-region fragments were found to be stably integrated. Furthermore, it has been established that co-transformation can occur with plant cells. Besides the detection of Ti-plasmid fragments from outside the T-region also DNA sequences originating from two DNA sources, which were both independently present in transformation experiments, have been found in some DNA transformants, e.g. calf thymus DNA, which was used as carrier DNA. No expression of the co-transferred DNA was observed. In total three phenotypical classes of DNA transformants were isolated. Although the T-DNA was often scrambled, polyA+ mRNA studies indicated that the different phenotypes studied can be explained by the presence of active T-DNA genes with known functions.
Similar content being viewed by others
References
Barker RF, Idler KB, Thompson DV, Kemp JD: Nucleotide sequence of the T-DNA region from the Agrobacterium tumefaciens octopine Ti plasmid pTi 15955. Plant Mol Biol 2:335–350, 1983.
Barry GF, Rogers SG, Fraley RT, Brand L: Identification of a cloned cytokinin biosynthetic gene. Proc Nat Acad Sci USA 81:4776–4780, 1984.
Bevan MW, Flavell RB, Chilton M-D: A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184–187, 1983.
Braun AC: The activation of two growth-substance systems accompanying the conversion of normal to tumor cells in crown gall. Cancer Res 16:53–56, 1956.
Chilton M-D, Tepfer DA, Petit A, David C, Casse-Delbart F, Tempé J: Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature 295:432–434, 1982.
Davey MR, Cocking EC, Freeman J, Pearce N, Tudor I: Transformation of Petunia protoplasts by isolated Agrobacterium plasmids. Pl Sci Lett 18: 307–313, 1980.
Fraley RT, Horsch RB, Matzke A, Chilton M-D, Chilton WS, Sanders PR: In vitro transformation of petunia cells by an improved method of co-cultivation with A. tumefaciens strains. Plant Mol Biol 3:371–378, 1984.
Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW: Genetic analysis of crown gall: fine structure map of T-DNA by site-directed mutagenesis. Cell 27:143–155, 1981.
Gelvin SB, Thomashow MF, McPherson JC, Gordon MP, Nester EW: Sizes and map positions of several plasmid-DNA-encoded transcripts in octopine-type crown gall tumors. Proc Nat Acad Sci USA 79:76–80, 1982.
Herrera-Estrella L, Depicker A, VanMontagu M, Schell J: Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303:209–213, 1983a.
Herrera-Estrella L, DeBlock M, Messens E, Hernalsteens J-P, VanMontagu M, Schell J: Chimeric genes as dominant selectable markers in plant cells. EMBO J 2:987–995, 1983b.
Hille J, Klasen I, Schilperoort RA: Construction and application of R-prime plasmids, carrying different segments of an octopine Ti-plasmid from Agrobacterium tumefaciens for complementation of vir genes. Plasmid 7:107–118, 1982.
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA: A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180, 1983.
Hoekema A, Roelvink PW, Hooykaas PJJ, Schilperoort RA: Delivery of T-DNA from the Agrobacterium tumefaciens chromosome into plant cells. EMBO J 3:2485–2490, 1984.
Holsters M, Villarroel R, Gielen J, Seurinck J, DeGreve H, VanMontagu M, Schell J: An analysis of the boundaries of the octopine T-DNA in tumors induced by Agrobacterium tumefaciens Mol Gen Genet 190:35–41, 1983.
Hooykaas-Van Slogteren GMS, Hooykaas PJJ, Schilperoort RA: Expression of Ti plasmid genes in monocotyledonous plant infected with Agrobacterium tumefaciens. Nature 311:763–764, 1984.
Hooykaas PJJ, Schilperoort RA: The molecular genetics of crown gall tumorigenesis. In: Scandalios JG (ed) Advances in genetics. Academic Press, New York, pp 209–283, 1983.
Iyer VN, Klee HJ, Nester EW: Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet 188:418–424, 1982.
Klee HJ, Gordon MP, Nester EW: Complementation analysis of Agrobacterium tumefaciens Ti-plasmid mutations affecting oncogenicity. J Bacteriol 150:327–331, 1982.
Klee HJ, White FF, Iyer VN, Gordon MP, Nester EW: Mutational analysis of the virulence region of an Agrobacterium tumefaciens Ti-plasmid. J Bacteriol 153:878–883, 1983.
Krens FA, Molendijk L, Wullems GJ, Schilperoort RA: In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature 296:72–74, 1982.
Krens FA, Wullems GJ, Schilperoort RA:Transformation of plant protoplasts in vitro. In: Ciferri O, Dure LIII (eds), Structure and function of plant genomes. Plenum Press, New York, pp 387–408, 1983.
Krens FA, Schilperoort RA: Ti-plasmid uptake and expression by protoplasts of Nicotiana tabacum. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, Volume I, Laboratory techniques. Academic Press, New York, 1984, pp 522–534.
Leemans J, Deblaere R, Willmitzer L, DeGreve H, Hernalsteens JP, VanMontagu M, Schell J: Genetic identification of functions of T-DNA transcripts in octopine crown galls. EMBO J 1:147–152, 1982.
Lemmers M, DeBeuckeleer M, Holsters M, Zambryski P, Depicker A, Hernalsteens JP, VanMontagu M, Schell J: Internal organization of Ti-plasmid DNA in nopaline crown gall tumors. J Mol Biol 144:353–376, 1980.
Linsmaier EM, Skoog F: Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127, 1965.
Márton L, Wullems GJ, Molendijk L, Schilperoort RA: In vitro transformation of cultured cells from Nicotiana tabacum by Agrobacterium tumefaciens. Nature 277:129–131, 1979.
Murai N, Kemp JD: T-DNA of pTi-15955 from Agrobacterium tumefaciens is transcribed into a minimum of seven polyadenylated RNAs in a sunflower crown gall tumor. Nucl Acids Res 10: 1679–1689, 1982.
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497, 1962.
Ooms G, Hooykaas PJJ, Molenaar G, Schilperoort RA: Crown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti-plasmids; analysis of T-DNA functions, Gene 14: 33–50, 1981.
Ooms G, Bakker A, Molendijk L, Wullems GJ, Gordon MP, Nester EW, Schilperoort RA: T-DNA organization in homogeneous and heterogeneous octopine-type crown gall tissues of Nicotiana tabacum. Cell 30: 589–597, 1982.
Paszkowski J, Shillito RD, Saul M, Mandák V, Hohn T, Hohn B, Potrykus I: Direct gene transfer to plants. EMBO J 3: 2717–2722, 1984.
Peerbolte R, Krens FA, Mans RMW, Floor M, Hoge JHC, Wullems GJ, Schilperoort RA: Transformation of plant protoplasts with DNA: cotransformation of non-selected calf thymus carrier DNA and meiotic segregation of transforming DNA sequences. Plant Mol Biol 5: 235–246, 1985.
Salomon F, Deblaere R, Leemans J, Hernalsteens JP, VanMontagu M, Schell J: Genetic identification of functions of TR-DNA transcripts in octopine crown galls. EMBO J 3: 141–146, 1984.
Schröder G, Waffenschmidt S, Weiler EW, Schröder J: The T-region of Ti-plasmids codes for an enzyme synthesizing indole 3-acetic acid. Eur J Biochem 138: 387–391, 1984.
Simpson RB, O'Hara PJ, Kwok W, Montoya AL, Lichtenstein C, Gordon MP, Nester EW: DNA from the A6S/2 crown gall tumor contains scrambled Ti-plasmid sequences near its junctions with plant DNA. Cell 29: 1005–1014, 1982.
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517, 1975.
Thomashow MF, Nutter R, Montoya AL, Gordon MP, Nester EW: Integration and organization of Ti-plasmid sequences in crown gall tumors. Cell 19: 729–739, 1980.
VanSlogteren GMS, Hoge JHC, Hooykaas PJJ, Schilperoort RA: Clonal analysis of heterogeneous crown gall tumor tissues induced by wild-type and shooter mutant strains of Agrobacterium tumefaciens-expression of T-DNA genes. Plant Mol Biol 2: 321–333, 1983.
Velten J, Willmitzer L, Leemans J, Ellis J, Deblaere R, VanMontagu M, Schell J: TR genes involved in agropine production. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Berlin, pp 303–312, 1983.
Wang K, Herrera-Estrella L, VanMontagu M, Zambryski P: Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from Agrobacterium to the plant genome. Cell 38: 455–462, 1984.
Willmitzer L, Simons G, Schell J: The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J 1: 139–146, 1982.
Wullems GJ, Molendijk L, Ooms G, Schilperoort RA: Differential expression of crown gall tumor markers in transformants obtained after in vitro Agrobacterium tumefaciens-induced transformation of cell wall regenerating protoplasts derived from Nicotiana tabacum. Proc Nat Acad Sci USA 78: 4344–4348, 1981.
Yadav NS, Vanderleyden J, Bennett DR, Barnes WM, Chilton M-D: Short direct repeats flank the T-DNA on a nopaline Ti plasmid. Proc Nat Acad Sci USA 79: 6322–6326, 1982.
Zambryski P, Depicker A, Kruger K, Goodman H: Tumor induction by Agrobacterium tumefaciens: analysis of the boundaries of T-DNA. J Mol App Genet 1: 361–370, 1982.
Zambryski P, Goodman HM, VanMontagu M, Schell J: Agrobacterium tumor induction. In: Shapiro JA (ed) Mobile genetic elements. Academic Press, New York, pp 505–535, 1983.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krens, F.A., Mans, R.M.W., van Slogteren, T.M.S. et al. Structure and expression of DNA transferred to tobacco via transformation of protoplasts with Ti-plasmid DNA: co-transfer of T-DNA and non T-DNA sequences. Plant Mol Biol 5, 223–234 (1985). https://doi.org/10.1007/BF00020640
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020640