Abstract
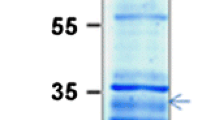
When 35%-acetone extract of spinach chloroplasts was separated by SDS-PAGE, ferredoxin-NADP reductase (FNR) appeared as a single band at a molecular mass of 35 kDa. After the polypeptides on the SDS-PAGE plate were electroblotted onto PVDF membrane, the FNR band was cut out and analyzed for N-terminal structure in a gas-phase protein sequencer. Two different FNR peptides were identified: one with glutamine at its N-terminus (Gln-FNR) and the other with γ-pyroglutamic acid (<Glu-FNR). Next, tightly bound FNR (tFNR) fraction was extracted from chloroplasts with their loosely bound FNR (lFNR) fraction removed in advance. The tFNR fraction contained Gln-FNR only. The Gln-FNR could be highly purified by affinity chromatography using a ferredoxin column. The purified Gln-FNR was digested with arginyl endopeptidase for peptide mapping and partial sequence analysis. Primary structure of Gln-FNR differed from that of <Glu-FNR previously reported by Karplus et al. (1984) only in that it has an unblocked N-terminus. Highly purified Gln-FNR gave a molecular mass of 35 kDa in SDS-PAGE analysis, but its apparent molecular mass was estimated to be 38.5 kDa by gel-filtration HPLC analysis. This larger apparent molecular mass of Gln-FNR could be ascribed to its N-terminal moiety with around 15 amino acid residues protruding outside of a globular FNR molecule.
Similar content being viewed by others
Abbreviations
- CBB:
-
Coomassie Brilliant Blue
- dabsyl chloride:
-
(dimethylamino)azobenzenesulfonyl chloride
- FNR:
-
ferredoxin-NADP reductase
- Gln-FNR:
-
FNR with glutamine at N-terminus
- Ile-FNR:
-
with isoleucine at N-terminus
- lFNR:
-
loosely bound FNR
- tFNR:
-
tightly bound FNR
- <Glu-FNR:
-
FNR with γ-pyroglutamic acid at N-terminus
References
Cockburn W, Walker DA and Baldry CW (1968) The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol 43: 1415–1418
Gadda G, Aliverti A, Ronchi S and Zannetti G (1990) Structure-function relationship in spinach ferredoxin-NADP+ reductase as studied by limited proteolysis. J Biol Chem 265: 11955–11959
Hewick RM, Hunkapiller MW, Hood LE and Dreyer WJ (1981) A gas-liquid solid phase peptide and protein sequencer. J Biol Chem 256: 7990–7996
Hirano H, Komatsu S, Nakamura A, Kikuchi F, Kajiwara H, Tsunasawa S and Sakiyama F (1991) Structural homology between semidwarfism-related proteins and gluterin seed protein in rice (Oryza sativa L.). Theor Appl Genet 83: 153–158
Jansen T, Reiländer H, Steppuhn J and Herrmann RG (1988) Analysis of cDNA clones encoding the entire precursor-polypeptide for ferredoxin: NADP+ oxidoreductase from spinach. Curr Genet 13: 517–522
Karplus PA, Daniels MJ and Herriott JR (1991) Atomic structure of ferredoxin-NADP+ reductase: Prototype for a structurally novel flavoenzyme family. Science 251: 60–66
Karplus PA, Walsh KA and Herriott JR (1984) Amino acid sequence of spinach ferredoxin:NADP+ oxidoreductase. Biochemistry 23: 6576–6583
Kuwabara T, Murata T, Miyao M and Murata N (1978) Prolinerich structure at amino-terminal region of the 18-kDa protein of photosynthetic oxygen-evolving complex. In: Biggins J (ed) Progress in Photosynthesis Research, Vol I, pp 705–708 Martinus Nijhoff Publishers, Dordrecht
Laemmli UK (1971) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680–685
Matsudaira P (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262: 10035–10038
Matsushita H, Kato I, Aoyama H, Tsunasawa S and Sakiyama F (1989) Arginyl endopeptidase. Tanpakushitu Kakusan Koso 34: 374–379 (in Japanese)
Matthijs HCP, Coughlan SJ and Hind G (1986) Removal of ferredoxin:NADP+ oxidoreductase from thylakoid membranes, rebinding to depleted membranes, and identification of the binding site. J Biol Chem 261: 12154–12158
Nishikawa T, Sakai K, Sakihama N and Shin M (1992) Identification of ferredoxin-NADP reductase located at some inner part of thylakoid membranes. In: Murata N (ed) Research in Photosynthesis, Vol II, pp 543–546. Kluwer Academic Publishers, Dordrecht/Boston/London
Obata S, Nishimura M, Nagai K, Sakihama N and Shin M (1995) Four ferredoxins from Japanese radish leaves. Arch Biochem Biophys 316: 797–802
Sakihama N, Nagai K, Ohmori H, Tomizawa H, Tsujita M and Shin M (1992) Immobilized ferredoxins for affinity chromatography of ferredoxin-dependent enzymes. J Chromatogr 597: 147–153
Sakihama N, Ohmori H, Sugimoto N, Yamasaki Y, Oshino R and Shin M (1983) Toyopearl HW-65C:ammonium sulfate as a new column chromatographic adsorbent for enzyme purification. J Biochem 93: 129–134
Sakihama N, Shin M and Obata S (1994) N-terminal structure of mature ferredoxin-NADP reductase In: Yagi K (ed) Flavins and Flavoproteins 1993, pp 439–442. Walter de Gruyter, Berlin/New York
Schägger H and vonJagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379
Sekido T, Sakihama N and Shin M (1992) The large form of ferredoxin-NADP reductase: Isolation and properties. In: Murata N (ed) Research in Photosynthesis, Vol II pp 547–550. Kluwer Academic Publishers, Dordrecht/Boston/London
Shin M, Tsujita M, Tomizawa H, Sakihama N, Kamei K and Oshino R (1990) Proteolytic degradation of ferredoxin-NADP reductase duing purification from spinach. Arch Biochem Biophys 279: 97–103
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakihama, N., Nishimura, I., Obata, S. et al. Mature ferredoxin-NADP reductase with a glutaminyl residue at N-terminus from spinach chloroplasts. Photosynth Res 46, 323–328 (1995). https://doi.org/10.1007/BF00020447
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020447