Abstract
A DNA fragment containing sequences hybridizing to the 5′ region of GS15, a gene encoding soybean cytosolic glutamine synthetase, was isolated from a soybean genomic library. Mapping and partial sequence analysis of the genomic clone revealed that it encodes a cytosolic GS gene, GS21, which is different from GS15.
In parallel, a number of cDNA clones encoding cytosolic GS were isolated using the coding region of pGS20 as a probe (pGS20 is a cDNA clone which corresponds to a transcript of the GS15 gene). Two new full-length cDNAs designated pGS34 and pGS38 were isolated and sequenced.
In the 5′ non-coding region a strong homology was found between the two clones and the GS21 gene. However, none of these sequences were identical, which suggests that there are at least three members in this group of genes.
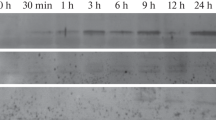
In order to determine their relative levels of transcription, specific sequences from pGS34, pGS38 and GS21 were used in an RNAse protection assay. This experiment clearly showed that GS21 and the gene encoding pGS38 are specifically expressed in young or mature nodules, whereas the gene encoding pGS34 is highly transcribed in nodules and constitutively expressed at a lower level in other soybean organs.
In order to further analyse the molecular mechanisms controlling GS21 transcription, different fragments of the promoter region were fused to the Escherichia coli reporter gene encoding β-glucuronidase (GUS) and the constructs were introduced into Lotus corniculatus via Agrobacterium rhizogenes-mediated transformation. Analysis of GUS activity showed that the GS21 promoter-GUS constructs were expressed in the vasculature of all vegetative organs. This result is discussed in relation to species-specific metabolic and developmental characteristics of soybean and Lotus.
Similar content being viewed by others
References
Becker TW, Caboche M, Carrayol E, Hirel B: Nucleotide sequence of a tobacco cDNA encoding plastidic glutamine synthetase and light inducibility, organ specificity and diurnal rythmicity in the expression of the corresponding genes of tobacco and tomato. Plant Mol Biol 19: 367–379 (1992).
Benfey PN, Chua NH: Regulated genes in transgenic plants. Science 244: 174–181 (1989).
Bennett MJ, Lighfoot DA, Cullimore JV: cDNA sequence and differential expression of the gene encoding the glutamine synthetase γ polypeptide of Phaseolus vulgaris L. Plant Mol Biol 12: 553–565 (1989).
Boron L, Legocki A: Cloning and characterization of a nodule-enhanced glutamine synthetase encoding gene from Lupinus luteus. Gene 136: 95–102 (1993).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Brears T, Walker EL, Coruzzi GM: A promoter sequence involved in cell-specific expression of the pea glutamine synthetase GS3A gene in organs of transgenic tobacco and alfalfa. Plant J 1: 235–244 (1991).
Cai X, Wong P: Subunit composition of glutamine synthetase isozymes from root nodules of bean (Phaseolus vulgaris L.). Plant Physiol 91: 1056–1062 (1989).
Coic Y, Lesaint C: Comment assurer une bonne nutrition en eau et en ions minéraux en horticulture. Hort Franç 8: 11–14 (1971).
Coic Y, Tendille J, Lesaint C: La nutrition azotée du tournesol (Helianthus annuus): action sur le rendement et la composition biochimique de la graine. Agrochimica 16: 254–263 (1972).
Coruzzi GM, Broglie R, Edwards C, Chua NM: Tissue specific and light regulated expression of a pea nuclear gene encoding small subunit of ribulose 1,5-bisphosphate carboxylase. EMBO J 3: 1671–1679 (1984).
Ditta G, Stanfiel S, Corbin D, Helinski DR: Broad host range DNA cloning system for Gram-negative bacteria: Construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77: 7347–7351 (1980).
Doyle JJ: Evolution of higher-plant glutamine synthetase genes: tissue specificity as a criterion for predicting orthology. Mol Biol Evol 8: 366–377 (1991).
Dunn K, Dickstein R, Feinbaum R, Burnett BK, Peterman TK, Thoidis G, Goodman HM, Ausubel FM: Developmental regulation of nodule-specific genes in alfalfa root nodules. Mol Plant-Microbe Interact 1: 66–74 (1988).
Edwards JW, Walker EL, Coruzzi GM: Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci USA 87: 3459–3463 (1990).
Forde BG, Cullimore JV: The molecular biology of glutamine synthetase in higher plants. Oxf Surv Plant Mol Cell Biol 6: 247–296 (1989).
Forde BG, Day HM, Turton JF, Wen-jun S, Cullimore JV, Oliver JE: Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. Plant Cell 1: 391–401 (1989).
Forde BG, Freeman J, Oliver JE, Pineda M: Nuclear factors interact with conserved A/T-rich elements upstream of a nodule-enhanced glutamine synthetase gene from french bean. Plant Cell 2: 925–939 (1990).
Gebhardt C, Oliver JE, Forde BG, Saarelainen R, Miflin BJ: Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J 5: 1429–1435 (1986).
Hirel B, Bouet C, King B, Layzell D, Jacobs F, Verma DPS: Glutamine synthetase genes are regulated by ammonia provided externally or by symbiotic nitrogen fixation. EMBO J 6: 1167–1171 (1987).
Hirel B, Miao GH, Verma DPS: Metabolic and developmental control of glutamine synthetase genes expression in legume and non-legume plants. In: Verma DPS (ed) Control of Plant Gene Expression, pp. 443–457. CRC Press, Boca Raton, FL (1992).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Jensen EO, Marcker KA, Schell J, de Bruijn F: Interaction of a nodule-specific, trans-acting factor with distinct DNA elements in the soybean leghaemoglobin lbc3 5′ upstream region. EMBO J 7: 1265–1271 (1988).
Joy KW: Ammonia, glutamine, and asparagine: a carbonnitrogen interface. Can J Bot 66: 2103–2109 (1988).
Kosugi S, Ohashi Y, Nakajima K, Arai Y: An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci. 70: 133–140 (1990).
Lea PJ, Miflin BJ: Transport and metabolism of asparagine and other nitrogen compounds within the plant. In: Miflin BJ (ed) The Biochemistry of Plants, vol. 5, pp. 569–607. Academic Press, New York (1980).
Lightfoot DA, Green NK, Cullimore JV: The chloroplastlocated glutamine synthetase of Phaseolus vulgaris L.: nucleotide sequence, expression in different organs and uptake into isolated chloroplasts. Plant Mol Biol 11: 191–202 (1988).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
McNally S, Hirel B: Glutamine synthetase isoforms in higher plants. Physiol Vég 21: 761–774 (1983).
Marsolier MC, Carrayol E, Hirel B: Multiple functions of promoter sequences involved in organ-specific expression and ammonia regulation of a cytosolic soybean glutamine synthetase gene in transgenic Lotus corniculatus. Plant J 3: 405–414 (1993).
Marsolier MC, Hirel B: Metabolic and developmental control of cytosolic glutamine synthetase genes in soybean. Physiol Plant 89: 613–617 (1993).
Miao GH, Hirel B, Marsolier MC, Ridge RW, Verma DPS: Ammonia-regulated expression of a soybean gene encoding cytosolic glutamine synthetase in transgenic Lotus corniculatus. Plant Cell 3: 11–22 (1991).
Miflin BJ, Lea PJ: Ammonia assimilation. In: Miflin BJ (ed), The Biochemistry of Plants, vol. 5, pp. 169–202. Academic Press, New York (1980).
Miflin BJ, Wallsgrove RM, Lea PJ: Glutamine metabolism in higher plants. In: Currents Topics in Cellular Regulation, vol. 20, pp. 1–39. Academic Press New York. (1981).
Moore HM, Nasrallah JB: A Brassica self-incompatibility gene is expressed in the stylar transmitting tissue of transgenic tobacco. Plant Cell 2: 29–38 (1990).
Murashige J, Skoog F: A revised medium for rapid growth and bio assay with tobacco tissue culture. Physiol Plant 115: 473–497 (1962).
Paszkowski J, Shillito RD, Saul M, Nandak V, Hohn T, Hohn B, Potrykus I: Direct gene transfer to plants. EMBO J 3: 2717–2722 (1984).
Petit A, Stougaard J, Kühle A, Marcker KA, Tempé J: Transformation and regeneration of the legume Lotus corniculatus: a system for molecular studies of symbiotic nitrogen fixation. Mol Gen Genet 207: 245–250 (1987).
Roche D, Temple SJ, Sengupta-Gopalan C: Two classes of differentially regulated glutamine synthetase genes are expressed in the soybean nodule: a nodule-specific class and a constitutively expressed class. Plant Mol Biol 22: 971–983 (1993).
Sanger F, Nicklen S, Coulsen A: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sengupta-Gopalan C, Pitas JW: Expression of nodule-specific glutamine synthetase genes during nodule development in soybeans. Plant Mol Biol 7: 189–199 (1986).
Stanford AC, Northcote DH, Bevan MW: Spatial and temporal patterns of transcription of a wound-induced gene in potato. EMBO J 9: 593–603 (1990).
Stougaard J, Sandal NN, Gron A, Kühle A, Marcker KA: Analysis of the soybean leghaemoglobin Ibc3 gene: regulatory elements required for promoter activity and organ specificity. EMBO J 6: 3565–3569 (1987).
Stougaard J, Jorgenson JE, Christensen T, Kühle A, Marcker KA: Interdependence and nodule specificity of cis-acting regulatory elements in the soybean leghemoglobin lbc3 and N23 gene promoters. Mol Gen Genet 220: 353–360 (1990).
Szabados L, Ratet P, Grunenberg R, de Bruijn F: Functional analysis of the Sesbania rostrata leghemoglobin glb3 gene 5′-upstream region in transgenic Lotus corniculatus and Nicotiana tabacum plants. Plant Cell 2: 973–986 (1990).
Tingey SV, Walker EL, Coruzzi GM: Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J 6: 1–9 (1987).
Tingey SV, Tsai FY, Edwards JW, Walker EL, Coruzzi GM: Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem 263: 9651–9657 (1988).
Tischer E, DasSarma S, Goodman HM: Nucleotide sequence of an alfalfa glutamine synthetase gene. Mol Gen Genet 203: 221–229 (1986).
Turton JF, Hopley AP, Forde BJ: 5′-flanking sequence of a glutamine synthetase gene specifying the β subunit of the cytosolic enzyme from Phaseolus vulgaris L. Nucl Acids Res 16: 11367 (1988).
Walker EL, Coruzzi GM: Developmentally regulated expression of the gene family for cytosolic glutamine synthetase in Pisum sativum. Plant Physiol 91: 702–708 (1989).
Wong SL, Verma DPS: Promoter analysis of a soybean nuclear gene coding for nodulin-23, a nodule-specific polypeptide. EMBO J 4: 2431–2437 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marsolier, MC., Debrosses, G. & Hirel, B. Identification of several soybean cytosolic glutamine synthetase transcripts highly or specifically expressed in nodules: expression studies using one of the corresponding genes in transgenic Lotus corniculatus . Plant Mol Biol 27, 1–15 (1995). https://doi.org/10.1007/BF00019174
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019174