Abstract
Mass spectrometric measurements of 16O2 and 18O2 isotopes were used to compare the rates of gross O2 evolution (E0), O2 uptake (U0) and net O2 evolution (NET) in relation to different concentrations of dissolved inorganic carbon (DIC) by Chlamydomonas reinhardtii cells grown in air (air-grown), in air enriched with 5% CO2 (CO2-grown) and by cells grown in 5% CO2 and then adapted to air for 6h (air-adapted).
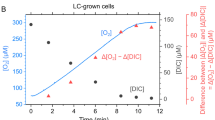
At a photon fluence rate (PFR) saturating for photosynthesis (700 μmol photons m-2 s-1), pH=7.0 and 28°C, U0 equalled E0 at the DIC compensation point which was 10μM DIC for CO2-grown and zero for air-grown cells. Both E0 and U0 were strongly dependent on DIC and reached DIC saturation at 480 μM and 70 μM for CO2-grown and air-grown algae respectively. U0 increased from DIC compensation to DIC saturation. The U0 values were about 40 (CO2-grown), 165 (air-adapted) and 60 μmol O2 mg Chl-1 h-1 (air-grown). Above DIC compensation the U0/E0 ratios of air-adapted and air-grown algae were always higher than those of CO2-grown cells. These differences in O2 exchange between CO2- and air-grown algae seem to be inducable since air-adapted algae respond similarly to air-grown cells.
For all algae, the rates of dark respiratory O2 uptake measured 5 min after darkening were considerably lower than the rates of O2 uptake just before darkening. The contribution of dark respiration, photorespiration and the Mehler reaction to U0 is discussed and the energy requirement of the inducable CO2/HCO3 - concentrating mechanism present in air-adapted and air-grown C. reinhardtii cells is considered.
Similar content being viewed by others
Abbreviations
- DIC:
-
dissolved inorganic carbon
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- E0 :
-
rate of photosynthetic gross O2 evolution
- PCO:
-
photosynthetic carbon oxidation
- PFR:
-
photon fluence rate
- PS I:
-
photosystem I
- PS II:
-
photosystem II
- U0 :
-
rate of O2 uptake in the light
- MS:
-
mass spectrometer
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 66: 407–413
Badger MR, Kaplan A and Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii. Plant Physiol 66: 407–413
Badger MR and Andrews TJ (1982) Photosynthesis and inorganic carbon usage by the marine Cyanobacterium, Synechococcus sp. Plant Physiol 70: 517–523
Badger MR (1985) Photosynthetic oxygen exchange. Ann Rev Plant Physiol 36: 27–53
Becker R and Fock HP (1983) Photosynthetic CO2 uptake and glycollate excretion of Chlamydomonas reinhardtii at normal and low O2 partial pressures as a function of the CO2 concentration. Photosynthetica 17: 51–58
Berry J, Boynton J, Kaplan A and Badger M (1976) Growth and photosynthesis of Chlamydomonas reinhardtii as a function of CO2 concentration. Carnegie Inst Year Book 75: 423–432
Berry JA, Osmond CB and Lorimer GH (1978) Fixation of 18O2 during photorespiration. Plant Physiol 62: 954–967
Canvin DT, Berry JA, Badger MR, Fock H and Osmond CB (1980) Oxygen exchange in leaves in the light. Plant Physiol 66: 302–307
Fock H, Canvin DT and Osmond CB (1981) Oxygen uptake in air-grown Chlamydomonas. In Akoyunoglou G (ed.) Photosynthesis IV, Regulation of Carbon Metabolism, pp 677–682. Philadelphia: Balaban International Science Services
Furbank RT and Badger MR (1982) Photosynthetic oxygen exchange in attached leaves of C4 monocotyledons. Aust J Plant Physiol 9: 553–558
Hill R (1939) Oxygen produced by isolated chloroplasts. Proc R Soc London Ser B 127 (847): 192–211
Kaplan A and Berry JA (1981) Glycolate excretion and the oxygen to carbon dioxide net exchange ratio during photosynthesis in Chlamydomonas reinhardtii. Plant Physiol 67: 229–232
Mehler AH (1951) Studies on reactions of illuminated chloroplasts II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys 34: 399–351
Ogawa T, Miyano A and Inoue Y (1985) Photosystem-I-driven inorganic carbon transport in the cyanobacterium Anacystis nidulans. Biochim Biophys Acta 808: 77–84
Peltier G and Thibault P (1985) Light dependent oxygen uptake, glycolate, and ammonia release in L-methionine sulfoximine treated Chlamydomonas. Plant Physiol 77: 281–284
Peltier G and Thibault P (1985) Oxygen uptake in the light in Chlamydomonas: Evidence for a persistent mitochondrial respiration. Plant Physiol 79: 225–230
Radmer RJ and Kok B (1976) Photoreduction of O2 primes and replaces CO2 assimilation. Plant Physiol 58: 336–340
Radmer RJ and Ollinger O (1980) Light-driven uptake of oxygen, carbon dioxide, and bicarbonate by the green alga, Scenedesmus. Plant Physiol 65: 723–729
Raven JA and Glidewell SM (1975) Sources of ATP for active phosphate transport in Hydrodictyon africanum: Evidence for a pseudocyclic photophosphorylation in vivo. New Phytol 75: 197–204
Ried A (1970) Energetic aspects of the interaction between photosynthesis and respiration. In: Prediction and Measurement of Photosynthetic Productivity, pp 231–246. Wageningen: PUDOC
Spalding MH and Ogren WL (1982) Photosynthesis is required for induction of the CO2-concentrating system in Chlamydomonas reinhardtii. FEBS Lett 145: 41–44
Spalding MH, Critchley C, Govindjee and Ogren WL (1984) Influence of carbon dioxide concentration during growth on fluorescence induction characteristics of the green alga Chlamydomonas reinhardtii. Photosynth Res 5: 169–176
Spalding MH and Portis AR (1985) A model of carbon dioxide assimilation in Chlamydomonas reinhardtii. Planta 164:308–320
Sültemeyer DF and Fock HP (1985) Mass spectrometric analysis of photosynthetic oxygen evolution and uptake by Chlamydomonas reinhardtii. In: Marcell R et al. (eds) Biological Control of Photosynthesis, pp 135–142. Dordrecht: Martinus Nijhoff
Sültemeyer DF, Fock HP and Klug K (1986) Effect of photon fluence rate on oxygen evolution and uptake by Chlamydomonas reinhardtii suspensions grown in ambient and CO2-grown enriched air. Plant Physiol 81: 372–375
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sültemeyer, D.F., Klug, K. & Fock, H.P. Effect of dissolved inorganic carbon on oxygen evolution and uptake by Chlamydomonas reinhardtii suspensions adapted to ambient and CO2-enriched air. Photosynth Res 12, 25–33 (1987). https://doi.org/10.1007/BF00019148
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019148