Abstract
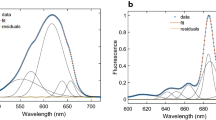
Fluorescence excitation spectra of highly anisotropic emission from Photosystem I (PS I) were measured at 295 and 77 K on a PS II-less mutant of the cyanobacterium Synechocystis sp. PCC 6803 (S. 6803). When PS I was excited with light at wavelengths greater than 715 nm, fluorescence observed at 745 nm was highly polarized with anisotropies of 0.32 and 0.20 at 77 and 295 K, respectively. Upon excitation at shorter wavelengths, the 745-nm fluorescence had low anisotropy. The highly anisotropic emission observed at both 77 and 295 K is interpreted as evidence for low-energy chlorophylls (Chls) in cyanobacteria at room temperature. This indicates that low-energy Chls, defined as Chls with first excited singlet-state energy levels below or near that of the reaction center, P700, are not artifacts of low-temperature measurements.
If the low-energy Chls are a distinct subset of Chls and a simple two-pool model describes the excitation transfer network adequately, one can take advantage of the low-energy Chls' high anisotropy to approximate their fluorescence excitation spectra. Maxima at 703 and 708 nm were calculated from 295 and 77 K data, respectively. Upper limits for the number of low-energy Chls per P700 in PS I from S. 6803 were calculated to be 8 (295 K) and 11 (77 K).
Similar content being viewed by others
Abbreviations
- Chl -:
-
chlorophyll
- BChl -:
-
bacteriochlorophyll
- LHC -:
-
light-harvesting chlorophyll
- PS -:
-
Photosystem
- RC -:
-
reaction center
- S. 6803 -:
-
Synechocystis sp. PCC 6803
References
Bassi R and Simpson D (1987) Chlorophyll-protein complexes of barley photosystem I. Eur J Biochem 163: 221–230
Bittersmann E and Vermaas W (1991) Fluorescence lifetime studies of cyanobacterial Photosystem mutants. Biochim Biophys Acta 1098: 105–116
Boekema EJ, Dekker JP, VanHeel MG, Rögner M, Saenger W, Witt I and Witt HT (1987) Evidence for a trimeric organization of the Photosystem I complex from the thermophilic cyanobacterium Synechococcus sp. FEBS Lett 217: 283–286
Bose S (1982) Chlorophyll fluorescence in green plants and energy transfer pathways in photosynthesis. Photochem Photobiol 36: 725–734
Butler WL (1961) A far-red absorbing form of chlorophyll, in vivo. Archiv Biochem Biophys 93: 413–422
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29: 345–378
Butler WL, Tredwell CJ, Malkin R and Barber J (1979) The relationship between the lifetime and yield of the 735 nm fluorescence of chloroplasts at low temperatures. Biochim Biophys Acta 545: 309–315
Cho F and Govindjee (1970) Low-temperature (4–77 K) spectroscopy of Anacystis; temperature dependence of energy transfer efficiency. Biochim Biophys Acta 216: 151–161
Danks SM, Evans EH and Whittaker PA (1983) Photosynthetic Systems: Structure, Function and Assembly. Wiley and Sons, New York
Förster T (1965) Delocalized excitation and excitation transfer. In: Sinanoslu O (ed) Modern Quantum Chemistry, Part III, pp 93–137. Academic Press, New York
French CS, Brown JS and Lawrence MC (1972) Four universal forms of chlorophyll a. Plant Physiol 49: 421–429
Friesner RA and Won Y (1989) Spectroscopy and electron transfer dynamics of the bacterial photosynthetic reaction center. Biochim Biophys Acta 977: 99–122
Garnier J, Maroc J and Guyon D (1986) Low-temperature fluorescence emission spectra and chlorophyll-protein complexes in mutants of Chlamydomonas reinhardtii: Evidence for a new chlorophyll-a-protein related to Photosystem I. Biochim Biophys Acta 851: 395–406
Goedheer JC (1964) Fluorescence bands and chlorophyll a forms. Biochim Biophys Acta 88: 304–317
Golbeck JH (1987) Structure, function and organization of the Photosystem I reaction center complex. Biochem Biophys Acta 895: 167–204
Golbeck JH (1992) Structure and function of Photosystem I. Annu Rev Plant Physiol Plant Mol Biol 43: 293–324
Golbeck JH and Bryant DA (1991) Photosystem I. In: Lee CP (ed) Current Topics in Bioenergetics, pp 167–204. Academic Press, New York
Gouterman M and Stryer L (1962) Fluorescence polarization of some porphyrins. J Chem Phys 37: 2260–2266
Hefti A, Ford RC, Miller M, Cox RP and Engel A (1992) Analysis of the structure of Photosystem I in cyanobacterial thylakoid membranes. FEBS Lett 296: 29–32
Holzwarth AR (1987) Picosecond fluorescence spectroscopy and energy transfer in photosynthetic antenna pigments. In: Barber J (ed) The Light Reactions, pp 95–157. Elsevier, Amsterdam
Holzwarth AR (1991) Excited-state kinetics in chlorophyll systems and its relationship to the functional organization of the photosystems. In: Scheer H (ed) Chlorophylls, pp 1125–1151. CRC Press, Boca Raton
Iwaki M, Mamoru M and Shigeru I (1992) Fluorescence of P700 and antenna chlorophylls in Photosystem I particles that contain 11 chlorophylls/P700. Biochim Biophys Acta 1100: 278–284
Jia Y, Jean JM, Werst MM, Chan CK and Fleming GR (1992) Simulations of the temperature dependence of energy transfer in the PS I core antenna. Biophys J 63: 259–273
Karukstis KK and Sauer K (1983) Fluorescence decay kinetics of chlorophyll in photosynthetic membranes. J Cell Biochem 23: 131–158
Knox RS (1977) Photosynthetic efficiency and excitation transfer and trapping. In: Barber J (ed) Primary Processes of Photosynthesis, Vol 2, pp 183–221. Academic Press, New York
Kramer HJM, Pennoyer JD, VanGrondelle R, Westerhuis WHJ, Niederman RA and Amesz J (1984) Low-temperature optical properties and pigment organization of the B875 light-harvesting bacteriochlorophyll-protein complex of purple photosynthetic bacteria. Biochim Biophys Acta 767: 335–344
Lakowicz JR (1983) Principles of Fluorescence Spectroscopy, Plenum Press, New York
Lam E, Ortiz W, Mayfield S and Malkin R (1984) Isolation and characterization of a light-harvesting chlorophyll a/b-protein complex associated with Photosystem I. Plant Physiol 74: 650–655
Lavorel J (1964) Héterogénéité de la chlorophylle in vivo: II. polarisation et spectres d'action de fluorescence. Biochim Biophys Acta 88: 20–36
Lyle PA and Struve WS (1991) Temperature dependence of antenna excitation transport in native Photosystem I particles. J Phys Chem 95: 4152–4158
Mimuro M (1988) Analysis of excitation energy transfer in thylakoid membranes by the time-resolved fluorescence spectra. In: Scheer H and Schneider S (eds) Photosynthetic Light Harvesting Systems, pp 589–600. W. de Gruyter, Berlin
Mohanty P, Zilinskas-Braun B, Govindjee and Thornber JP (1972) Chlorophyll fluorescence characteristics of system I chlorophyll a-protein complex and system II particles at room and liquid nitrogen temperatures. Plant Cell Physiol 13: 81–91
Moya I, Mullet JE, Briantais JM and Garcia R (1981) Comparison between lifetime spectra of chloroplasts and subchloroplast particles at −196 and 20°C. In: Akoyunoglou G (ed) Proc 5th Intl Congr Photosynthesis, pp 163–172. Balaban Int Sci Services, Philadelphia
Mukerji I and Sauer K (1989) Temperature-dependent steady-state and picosecond kinetic fluorescence measurements of a Photosystem I preparation from spinach. In: Briggs WH (ed) Photosynthesis, pp 105–122. Liss, New York
Mukerji I and Sauer K (1990) A spectroscopic study of a Photosystem I antenna complex. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol 2, pp 321–324. Kluwer Academic Publishers, Dordrecht
Mullet JE, Burke JJ and Arntzen CJ (1980) Chlorophyll proteins of Photosystem I. Plant Physiol 65: 814–822
Nechushtai R, Nourizadeh SD and Thornber JP (1986) A re-evaluation of the fluorescence of the core chlorophylls of Photosystem I. Biochim Biophys Acta 848: 193–200
Owens TG, Webb SP, Alberte RS, Mets L and Fleming GR (1988) Antenna structure and excitation dynamics in Photosystem I. I. Studies of detergent-isolated Photosystem I preparations using time-resolved fluorescence analysis. Biophys J 53: 733–745
Owens TG, Webb SP, Mets L, Alberte RS and Fleming GR (1989) Antenna structure and excitation dynamics in Photosystem I. II. Studies with mutants of Chlamydomonas reinhardtii lacking photosystem II. Biophys J 56: 95–106
Rijgersberg CP and Amesz J (1980) Fluorescence and energy transfer in phycobiliprotein-containing algae at low temperature. Biochim Biophys Acta 593: 261–271
Rijgersberg CP, Amesz J, Thielen APGM and Swager JA (1979) Fluorescence emission spectra of chloroplasts and subchloroplast preparations at low temperature. Biochim Biophys Acta 545: 473–482
Rögner M, Nixon PJ and Diner BA (1990) Purification and characterization of PS I and PS II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem 265: 6189–6196
Satoh K and Butler WL (1978a) Competition between the 735 nm fluorescence and the photochemistry of Photosystem I in chloroplasts at low temperature. Biochim Biophys Acta 502: 103–110
Satoh K and Butler WL (1978b) Low temperature spectral properties of subchloroplast fractions purified from spinach. Plant Physiol 61: 373–379
Scheer H and Schneider S (eds) (1988) Photosynthetic Light Harvesting Systems. W. de Gruyter, Berlin
Searle GFW, Tamkivi R, VanHoek A and Schaafsma TJ (1988) Temperature dependence of antennae chlorophyll fluorescence kinetics in Photosystem I reaction centre protein. J Chem Soc, Faraday Trans 2, 84: 315–327
Shubin VV, Murthy SDS, Karapetyan NV, Mohanty P (1991) Origin of the 77 K fluorescence at 758 nm in the cyanobacterium Spirulina platensis. Biochim Biophys Acta 1060: 28–36
Tabbutt S (1987) Spectroscopic studies of energy transfer in photosynthetic reaction centers of higher plants. PhD Thesis, University of California, Berkeley, CA
Takahashi Y, Koike H and Katoh S (1982) Multiple forms of chlorophyll-protein complexes from a thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys 219: 209–218
Tapie P, Choquet Y, Breton J, Delepelaire P and Wollman FA (1984) Orientation of Photosystem I pigments: Investigation by low-temperature linear dichroism and polarized fluorescence emission. Biochim Biophys Acta 767: 57–69
Turconi S, Schweitzer G and Holzwarth AR (1993) Temperature dependence of picosecond fluorescence kinetics of a cyanobacterial photosystem I particle. Photochem Photobiol 57: 113–119
Tusov VB, Korvatovskii BN, Pashchenko VZ and Rubin AB (1980). Nature of 735-nm fluorescence of chloroplasts at room and low temperatures. Doklady-biophysics [Eng trans] 252: 112–115
VanDorssen RJ, Vasmel H and Amesz J (1985) Antenna organization and energy transfer in membranes of Heliobacterium chlorum. Biochim Biophys Acta 809: 199–203
VanGrondelle R (1985) Excitation energy transfer, trapping and annihilation in photosynthetic systems. Biochim Biophys Acta 811: 147–195
VanGrondelle R and Sundström V (1988) Excitation energy transfer in photosynthesis. In: Scheer H and Schneider S (eds) Photosynthetic Light Harvesting Systems, pp 403–438. W. de Gruyter, Berlin
VanGrondelle R, Bergstrom H, Sundström V, VanDorssen RJ, Vos M and Hunter CN (1988) Excitation energy transfer in the light-harvesting antenna of photosynthetic purple bacteria: the role of the long-wavelength absorbing pigment B896. In: Scheer H and Schneider S (eds) Photosynthetic Light Harvesting Systems, pp 519–530. W. de Gruyter, Berlin
VanMetter RL (1977) Excitation energy transfer in the light-harvesting chlorophyll a/b-protein. Biochim Biophys Acta 462: 642–658
VanMourik F, Visschers RW and VanGrondelle R (1992) Energy transfer and aggregate size effects in the inhomogeneously broadened core light-harvesting complex of Rhodobacter sphaeroides. Chem Phys Letts 193: 1–7
VanMourik F, Visscher KJ, Mulder JM and VanGrondelle R (1993) Spectral inhomogeneity of the light-harvesting antenna of Rhodospirillum rubrum probed by triplet-minus-singlet spectroscopy and singlet-triplet annihilation at low temperatures. Photochem Photobiol 57: 19–23
Vermaas WFJ, Williams JGK and Arntzen CJ (1987) Sequencing and modification of psbB, the gene encoding the CP-47 protein of Photosystem II, in the cyanobacterium Synechocystis 6803. Plant Mol Biol 8: 317–326
Vermaas WFJ, Ikeuchi M and Inoue Y (1988) Protein composition of the Photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res 17: 97–113
Vermaas WFJ, Charité J and Shen G (1990) Glu-69 of the D2 protein in Photosystem II is a potential ligand to Mn involved in photosynthetic oxygen evolution. Biochemistry 29: 5325–5332
Visschers RW, Chang MC, VanMourik F, Parkes-Loach PS, Heller BA, Loach PA and VanGrondelle R (1991) Fluorescence polarization and low-temperature absorption spectroscopy of a subunit form of light-harvesting complex I from purple photosynthetic bacteria. Biochemistry 30: 5734–5742
Werst M, Jia Y, Mets L and Fleming GR (1992) Energy transfer and trapping in the Photosystem I core antenna. Biophys J 61: 868–878
Wittmershaus BP (1987) Measurements and kinetic modeling of picosecond time-resolved fluorescence from Photosystem I and chloroplasts. In: Biggins J (ed) Progress in Photosynthesis Research, Vol 1, pp 75–82. Martinus Nijhoff/Dr W. Junk Publishers, Dordrecht
Wittmershaus BP, Berns DS and Huang C (1987) Picosecond time-resolved fluorescence from detergent-free Photosystem I particles. Biophys J 52: 829–836
Wittmershaus BP, Woolf VM and Vermaas WFJ (1992) Temperature dependence and polarization of fluorescence from Photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res 31: 75–87
Wollman FA and Bennoun P (1982) A new chlorophyll-protein complex related to Photosystem I in Chlamydomonas reinhardtii. Biochim Biophys Acta 680: 352–360
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Woolf, V.M., Wittmershaus, B.P., Vermaas, W.F.J. et al. Resolution of low-energy chlorophylls in Photosystem I of Synechocystis sp. PCC 6803 at 77 and 295 K through fluorescence excitation anisotropy. Photosynth Res 40, 21–34 (1994). https://doi.org/10.1007/BF00019042
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019042