Abstract
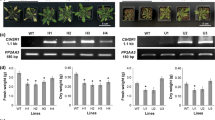
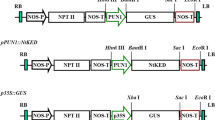
The tomato anionic peroxidase genes (tap1 and tap2) are induced by wounding and pathogen attack. The 5′-flanking region of tap1 confers wound- and pathogen-inducible β-glucuronidase (GUS) expression in tobacco plants transformed with a tap1/GUS chimeric fusion gene construct. A series of nested 5′ promoter deletions in the tap1/GUS fusion gene construct was created, and introduced into tobacco protoplasts via polyethylene glycol-mediated DNA transfer. A −202 construct (where the transcriptional start site is denoted +1) and larger tap1 promoter constructs showed constitutive GUS expression. A 2-fold increase in GUS expression over the high constitutive levels was observed with −358 bp and larger tap1 constructs when protoplasts were incubated with elicitor preparations from Verticillium albo-atrum. In tobacco plants transformed with the tap1 promoter deletion/GUS fusion gene constructs, wounding caused induction of GUS expression by 20 h that increased 6- to 18-fold by 72 h. The region between −202 and −358 of the tap1 promoter conferred wound responsiveness. GUS was also found to be expressed in the epidermis and trichomes in the aerial parts of transgenic plants. High-level GUS expression was observed in the nodal region of stems that was associated with the leaf traces. GUS that was absent in very young flower buds was found in the subsequent developmental stages in the pistils, ovaries and anthers. The developmentally regulated tissue-specific expression of GUS was found with all constructs containing the −202 and larger promoters whereas wound and pathogen induction required −358 or larger promoter. These results suggest that the tap1 gene, which was heretofore thought to be expressed only upon wounding or pathogen attack, plays a role in normal developmental processes of the plant and this gene acquired additional 5′-flanking promoter for the purpose of responding to wounding and fungal attack.
Similar content being viewed by others
References
Armstrong GA, Weisshaar B, Hahlbrock K: Homodimeric and heterodimeric leucine zipper proteins and nuclear factors from parsley recognize diverse promoter elements with ACGT cores. Plant Cell 4: 525–537 (1992).
Bevan M, Shufflebottom D, Edwards K, Jefferson RA, Schuch W: Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. Embo J 8: 1899–1906 (1989).
Borchert R: Time-course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol 62: 789–792 (1978).
Bowles DJ: Defense-related proteins in higher plants. Annu Rev Biochem 59: 873–907 (1990).
Brough CL, Sunter G, Gardiner WE, Bisaro DM: Kinetics of tomato golden mosaic virus DNA replication and coat protein promoter activity in Nicotiana tabacum protoplasts. Virology 187: 1–9 (1991).
Bustos MM, Begum D, Kalkan FA, Battraw MJ, Hall TC: Positive and negative cis-acting DNA domains are required for spatial and temporal regulation of gene expression by a seed storage protein promoter. Embo J 10: 1469–1479 (1991).
Côté F, Cutt JR, Asselin A, Klessig DF: Pathogenesis-related acidic β-1,3-glucanase genes of tobacco are regulated by both stress and developmental signals. Mol Plant-Microbe Interact 4: 173–181 (1991).
delCampillo E, Lewis RN: Occurrence of 9.5 cellulase and other hydrolases in flower reproductive organs undergoing major cell wall disruption. Plant Physiol 99: 1015–1020 (1992).
delCampillo E, Reid PD, Sexton R, Lewis RN: Occurrence and localization of 9.5 cellulase in abscising and non abscising tissues. Plant Cell 2: 245–254 (1990).
Driouich A, Laine A-C, Vian B, Faye L: Characterization and localization of laccase forms in stem and cell cultures of sycamore. Plant J 2: 13–24 (1992).
Esau K: In: Anatomy of Seed Plants, 2nd edn., pp. 61–70; John Wiley, New York (1977).
Fish TM, Prywes R, Simon MC, Roeder RG: Multiple sequence elements in the c-fos promoter mediate induction by cAMP. Genes Devel 3, 198–211 (1989).
Fry SC: Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37: 165–186 (1986).
Giuliano G, Pichersky E, Malik VS, Timko MP, Scolinik PA, Cashmore AR: An evolutionary conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA 85: 7089–7093 (1988).
Goldberg R, Imberty A, Liberman M, Prat R: Relationships between peroxidatic activities and cell wall plasticity. In: Greppin H, Penel C, Gaspar Th (eds) Molecular and Physiological Aspects of Plant Peroxidases, pp. 209–220. Université de Genève, Genève (1986).
Guiltinan MJ, MarcotteJr WR, Quatrano RH: A plant leucine zipper protein that recognizes an abscisic acid response element. Science 250: 267–271 (1990).
Harkin JM, Obst JR: Lignification in trees: indication of exclusive peroxidase participation. Science 180: 296–298 (1973).
Horsch RB, Fry J, Hoffmann N, Neidermeyer J, Rogers SG, Fraley RT: Leaf disc transformation. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant Molecular Biology Manual, pp. A5–1-A5–9. Kluwer Academic Publishers, Dordrecht, Netherlands (1988).
Jacobsen JV, Close TJ: Control of transient expression of chimeric genes by gibberellic acid and abscisic acid in protoplasts prepared from mature barley aleurone layers. Plant Mol Biol 16: 713–724 (1991).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Katagiri F, Lam E, Chua N-M: Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature 340: 727–730 (1989).
Keller B, Schmid J, Lamb CJ: Vascular expression of a bean cell wall glycine-rich protein-β-glucuronidase gene fusion in transgenic tobacco. EMBO J 8: 1309–1314 (1989).
Kolattukudy PE: Biopolyester membranes of plants: cutin and suberin. Science 208: 990–1000 (1980).
Kolattukudy PE: Cutin, suberin, and waxes. In: Stumpf PK, Conn EE (eds) The Biochemistry of Plants, vol. 4, pp. 571–644. Academic Press, New York (1980).
Kolattukudy PE, Mohan R, Bajar MA, Sherf BA: Plant peroxidase gene expression and function. In: Boryer S (ed.) Plant Oxygenases, Peroxidases and Oxidases. Biochemical Society Transactions vol. 20, pp. 333–337.
Kononowicz AK, Nelson DE, Singh NK, Hasegawa PM, Bressan RA: Regulation of the osmotin gene promoter. Plant Cell 4: 513–524 (1992).
Leyva A, Liang X, Pintor-toro JA, Dixon RA, Lamb CJ: Cis-element combinations determine phenylalanine ammonia-lyase gene tissue-specific expression patterns. Plant Cell 4: 263–271 (1992).
Liang X, Dron M, Schmid J, Dixon R, Lamb CJ: Developmental and environmental regulation of phenylalanine ammonia-lyase-β-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci USA 86: 9284–9288 (1989).
Lotan T, Ori N, Fluhr R: Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1: 881–887 (1989).
MacAdam JW, Sharp RE, Nelson CJ: Peroxidase activity in the leaf elongation zone of tall fescue. I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99: 872–878 (1992).
MacAdam JW, Sharp RE, Nelson CJ: Peroxidase activity in the leaf elongation zone of tall fescue. II. Spatial distribution of apoplastic peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99: 879–885 (1992).
Maniatis T, Fritsch FE, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
Mohan R, Kolattukudy PE: Differential activation of expression of a suberization-associated anionic peroxidase in near-isogenic resistant and susceptible tomato lines by elicitors of Verticillium albo-atrum. Plant Physiol 92: 276–280 (1990).
Mohan R, Bajar AM, Kolattukudy PE: Induction of a tomato anionic peroxidase gene (tap1) by wounding in transgenic tobacco and activation of tap1/GUS and tap2/GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Mol Biol 21: 339–354 (1993).
Neale AD, Wahleithner JA, Lund M, Bonnet HT, Kelly A, Wagner DRM, Peacock WJ, Dennis ES: Chitinase, β-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell 2: 673–684 (1990).
Negrutiu I, Shillito R, Potrykus I, Biasini G, Sala F: Hybrid genes in the analysis of transformation conditions. I. Setting up a simple method for direct gene transfer in plant protoplasts. Plant Mol Biol 8: 363–373 (1987).
Riley R, Kolattukudy PE: Evidence for covalently attached p-coumaric acid and ferulic acid in cutins and suberins. Plant Physiol 56: 650–654 (1975).
Robb J, Lee SW, Mohan R, Kolattukudy PE: Chemical characterization of stress-induced vascular coating. Plant Physiol 97: 528–536 (1991).
Roberts E, Kolattukudy PE: Molecular cloning, nucleotide sequence and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet 217: 223–231 (1989).
Roberts E, Kutchan T, Kolattukudy PE: Cloning and sequencing of cDNA for a highly anionic peroxidase from potato and the induction of its mRNA in suberizing potato tubers and tomato fruits. Plant Mol Biol 11: 15–26 (1988).
Sherf B, Kolattukudy PE: Developmentally regulated expression of the wound- and pathogen-responsive tomato anionic peroxidase in green fruits. Plant J, in press.
Showalter AM, Varner JE: Plant hydroxyproline-rich glycoproteins. In: Marcus A (ed.) The Biochemistry of Plants: A Comprehensive Treatise, vol. 11: Molecular Biology, pp. 485–520. Academic Press, New York (1989).
Siebertz B, Logemann J, Willmitzer L, Schell J: Cis-analysis of the wound-inducible promoter wun1 in transgenic tobacco plants and histochemical localization of its expression. Plant Cell 1: 961–968 (1989).
Soliday CL, Dean BB, Kolattukudy PE: Suberization: Inhibition by washing and stimulation by abscisic acid in potato disks and tissue culture. Plant Physiol 61: 170–174 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohan, R., Vijayan, P. & Kolattukudy, P.E. Developmental and tissue-specific expression of a tomato anionic peroxidase (tap1) gene by a minimal promoter, with wound and pathogen induction by an additional 5′-flanking region. Plant Mol Biol 22, 475–490 (1993). https://doi.org/10.1007/BF00015977
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00015977