Abstract
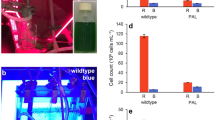
Part of the chlL gene encoding a component involved in light-independent protochlorophyllide reduction was deleted in wild type and in a photosystem I-less strain of Synechocystis sp. PCC 6803. In resulting mutants, chlorophyll biosynthesis was fully light-dependent. When these mutants were propagated under light-activated heterotrophic growth conditions (in darkness except for 15 min of weak light a day) for several weeks, essentially no chlorophyll was detectable but protochlorophyllide accumulated. Upon return of the chlL - mutant cultures to continuous light, within the first 6 h chlorophyll was synthesized at the expense of protochlorophyllide at a rate independent of the presence of photosystem I. Chlorophyll biosynthesized during this time gave rise to a 685 nm fluorescence emission peak at 77 K in intact cells. This peak most likely originates from a component different from those known to be directly associated with photosystems II and I. Development of 695 and 725 nm peaks (indicative of intact photosystem II and photosystem I, respectively) required longer exposures to light. After 6 h of greening, the rate of chlorophyll synthesis slowed as protochlorophyllide was depleted. In the chlL - strain, greening occurred at the same rate at two different light intensities (5 and 50 μE m-2s-1), indicating that also at low light intensity the amount of light is not rate-limiting for protochlorophyllide reduction. Thus, in this system the rate of chlorophyll biosynthesis is limited neither by biosynthesis of photosystems nor by the light-dependent protochlorophyllide reduction. We suggest the presence of a chlorophyll-binding ‘chelator’ protein (with 77 K fluorescence emission at 685 nm) that binds newly synthesized chlorophyll and that provides chlorophyll for newly synthesized photosynthetic reaction centers and antennae.
Similar content being viewed by others
References
Adamska I, Kloppstech K: Evidence for an association of the early light-inducible protein (ELIP) of pea with photosystem II. Plant Mol Biol 16: 209–222 (1991).
Adamska I, Ohad I, Kloppstech K: Synthesis of the early light-inducible protein is controlled by blue light and related to light stress. Proc Natl Acad Sci USA 89: 2610–2613 (1992).
Anderson SL, McIntosh L: Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: A blue-light-requiring process. J Bact 173: 2761–2767 (1991).
Bauer CE, Bollivar DW, Suzuki JY: Genetic analysis of photopigment biosynthesis in eubacteria: a guiding light for algae and plants. J Bact 175: 3919–3925 (1993).
Beale SI, Weinstein JD: Biochemistry and regulation of photosynthetic pigment formation in plants and algae. In: Jordan PM (ed) Biosynthesis of Tetrapyrroles, pp. 155–235. Elsevier, Amsterdam (1991).
Beale SI: Biosynthesis of cyanobacterial tetrapyrrole pigments: hemes, chlorophylls, and phycobilins. In: Bryant DA (ed) The Molecular Biology of Cyanobacteria, pp. 519–558. Kluwer Academic Publishers, Dordrecht (1994).
Bennett J: Biosynthesis of the light-harvesting chlorophyll a/b protein: polypeptide turnover in darkness. Eur J Biochem 118: 61–70 (1981).
Burke DH, Alberti M, Hearst JE: bchFNBH bacteriochlorophyll synthesis genes of Rhodobacter capsulatus and identification of the third subunit of light-independent protochlorophyllide reductase in bacteria and plants. J Bact 175: 2414–2422 (1993).
Burnap RL, Troyan T, Sherman LA: The highly abundant chlorophyll-protein complex of iron-deficient Synechococcus sp. PCC 7942 (CP43′) is encoded by the isiA gene. Plant Physiol 103: 893–902 (1993).
Carpenter SD, Vermaas WFJ: Directed mutagenesis to probe the structure and function of photosystem II. Physiol Plant 77: 436–443 (1989).
Darrah PM, Kay SA, Teakle GR, Griffiths WT: Cloning and sequencing of protochlorophyllide reductase. Biochem J 265: 789–798 (1990).
Dolganov NAM, Bhaya D, Grossman AR: Cyanobacterial protein with similarity to the chlorophyll a/b-binding proteins of higher plants: evolution and regulation. Proc Natl Acad Sci USA 92: 636–640 (1995).
Elhai J, Wolk CP: A versatile class of positive-selection vectors based on the non-viability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68: 119–138 (1988).
Eichacker L, Paulsen H, Rüdiger W: Synthesis of chlorophyll a regulates translation of chlorophyll a apoproteins P700, CP43, CP47, and D2 in barley etioplasts. Eur J Biochem 205: 17–24 (1992).
Fujita Y, Takahashi Y, Chuganji M, Matsubara H: The nifH-like (frxC) gene is involved in the biosynthesis of chlorophyll in the filamentous cyanobacterium Plectonema boryanum. Plant Cell Physiol 33: 81–92 (1992).
Fujita Y, Matsumoto H, Takahashi Y, Matsubara H: Identification of a nifDK-like gene (ORF467) involved in the biosynthesis of chlorophyll in the cyanobacterium Plectonema boryanum. Plant Cell Physiol 34: 305–314 (1993).
Griffiths WT: Protochlorophyllide photoreduction. In: Scheer H (ed) Chlorophylls, pp. 433–449. CRC Press, Boca Raton, FL (1991).
Haag E, Eaton-Rye JJ, Renger G, Vermaas WFJ: Functionally important domains of the large hydrophilic loop of CP47 as probed by oligonucleotide-directed mutagenesis in Synechocystis sp. PCC 6803. Biochemistry 32: 4444–4454 (1993).
Kim J, Klein PG, Mullet JE: Ribosomes pause at specific sites during synthesis of the membrane-bound chloroplast reaction center protein D1. J Biol Chem 266: 14931–14938 (1991).
Kim J, Eichacker LA, Rüdiger W, Mullet JE: Chlorophyll regulates accumulation of the plastid-encoded chlorophyll proteins P700 and D1 by increasing apoprotein stability. Plant Physiol 104: 907–916 (1994).
Laudenbach DE, Straus NA: Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J Bact 170: 5018–5026 (1988).
Li J, Goldschmidt-Clermont M, Timko MP: Chloroplastencoded chlB is required for light-independent protochlorophyllide reductase in Chlamydomonas reinhardtii. Plant Cell 5: 1817–1829 (1993).
Lidholm J, Gustafsson P: Homologues of the green algal gidA gene and the liverwort frxC gene are present on the chloroplast genomes of conifers. Plant Mol Biol 17: 787–798 (1991).
Liu XQ, Xu H, Huang C: Chloroplast chlB gene is required for light-independent chlorophyll accumulation in Chlamydomonas reinhardtii. Plant Mol Biol 23: 297–308 (1993).
MacKinney G: Absorption of light by chlorophyll solution J Biol Chem 140: 315–322 (1941).
Nakatani HY, Ke B, Dolan E, Arntzen CJ: Identity of the photosystem II reaction center polypeptide. Biochim Biophys Acta 765: 347–352 (1984).
Ogura Y, Takemura M, Yamato K, Ohta E, Fukuzawa H, Ohyama K: Cloning and nucleotide sequence of a frxC-ORF469 gene cluster of Synechocystis sp. PCC 6803: conservation with liverwort chloroplast frxC-ORF465 and nif operon. Biosci Biotechnol Biochem 56: 788–793 (1992).
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H: Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322: 572–574 (1986).
Pakrasi HB, Vermaas WFJ: Protein engineering of photosystem II. In: Barber J (ed) The Photosystems: Structure, Function and Molecular Biology. Current Topics in Photosystems, vol 11, pp. 231–256. Elsevier, Amsterdam (1992).
Richards WR: Biosynthesis of the chlorophyll chromophore of pigmented thylakoid proteins. In: Sundqvist C, Ryberg M (eds) Pigment-Protein Complexes in Plastids: Synthesis and Assembly, pp. 91–178. Academic Press, San Diego (1993).
Riethman HC, Sherman LA: Immunological characterization of iron-regulated membrane proteins in the cyanobacterium Anacystis nidulans R2. Plant Physiol 88: 497–505 (1988).
Riethman HC, Sherman LA: Purification and characterization of an iron stress-induced chlorophyll-protein from the cyanobacterium Anacystis nidulans R2. Biochim Biophys Acta 935: 141–151 (1988).
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY: Genetic assignment, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61 (1979).
Ryberg M, Sundqvist C: Structural and functional significance of pigment-protein complexes of chlorophyll precursors. In: Scheer H (ed) Chlorophylls, pp. 587–612. CRC Press, Boca Raton, FL (1991).
Schulz R, Steinmüller K, Klaas M, Forreiter C, Rasmussen S: Nucleotide sequece of cDNA coding for the NADPH-protochlorophyllide oxidoreductase (PCR) of barley (Hordeum vulgare L.) and its expression in Escherichia coli. Mol Gen Genet 217: 355–361 (1989).
Senge MO: Recent advances in the biosynthesis and chemistry of chlorophylls. Photochem Photobiol 57: 189–206 (1993).
Shen G, Boussiba S, Vermaas WFJ: Synechocystis sp. PCC 6803 strains lacking photosystem I and phycobilisome function. Plant Cell 5: 1853–1863 (1993).
Shen G, Vermaas WFJ: Mutation of chlorophyll ligands in the chlorophyll-binding CP47 protein as studied in a Synechocystis sp. PCC 6803 photosystem I-less background. Biochemistry 33: 7379–7388 (1994).
Shen G, Vermaas WFJ: Chlorophyll in a Synechocystis sp. PCC 6803 mutant without photosystem I and phtosystem II core complexes: evidence for peripheral antenna chlorophylls in cyanobacteria. J Biol Chem 269: 13904–13910 (1994).
Smart LB, Anderson SL, McIntosh L: Targeted genetic inactivation of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC 6803. EMBO J 10: 3289–3296 (1991).
Suzuki JY, Bauer CE: Light-independent chlorophyll biosynthesis: involvement of the chloroplast gene chlL (frxC). Plant Cell 4: 929–940 (1992).
van Dorssen RJ, Breton J, Plijter JJ, Satoh K, van Gorkom HJ, Amesz J: Spectroscopic properties of the reaction center and of the 47 kDa chlorophyll protein of photosystem II. Biochim Biophys Acta 893: 267–274 (1987).
Vermaas WFJ: Molecular-biological approaches to analyze photosystem II structure and function. Annu Rev Plant Physiol Plant Mol Biol 44: 457–481 (1993).
Vermaas WFJ, Carpenter S, Bunch C: Specific mutagenesis as a tool for the analysis of structure/function relationships in photosystem II. In: Singhal GS, Barber J, Dilley RA, Govindjee, Haselkorn R, Mohanty P (eds) Photosynthesis: Molecular Biology and Bioenergetics, pp. 21–35. Narosa, New Delhi (1989).
Vermaas WFJ, Ikeuchi M, Inoue Y: Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res 17: 97–113 (1988).
Vermaas WFJ, Williams JGK, Arntzen CJ: Sequencing and modification of psbB, the gene encoding CP-47 protein of photosystem II, in the cyanobacterium Synechocystis 6803. Plant Mol Biol 8: 317–326 (1987).
Vermaas WFJ, Williams JGK, Rutherford AW, Mathis P, Arntzen CJ: Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci USA 83: 9474–9477 (1986).
Williams JGK: Construction of specific mutations in the photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Meth Enzymol 167: 766–778 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, Q., Vermaas, W.F.J. Light-dependent chlorophyll a biosynthesis upon chlL deletion in wild-type and photosystem I-less strains of the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol 29, 933–945 (1995). https://doi.org/10.1007/BF00014967
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00014967