Abstract
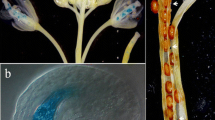
A genomic clone for the cyc07 gene, which is expressed specifically at the S phase during the cell cycle in synchronous cultures of periwinkle (Catharanthus roseus) cells, was isolated. Determination of the nucleotide sequence of the clone revealed that the cyc07 gene consists of seven exons separated by six introns. Genomic Southern analysis indicated that the cyc07 gene is present as a single copy per haploid genome in periwinkle. Expression of related genes was detected in a wide range of other plants. Transgenic Arabidopsis plants were generated that expressed the gene for β-glucuronidase (GUS) under the control of the promoter of the cyc07 gene. The tissue-specific pattern of expression directed by the promoter was investigated by analysis of GUS activity. Histochemical tests demonstrated that 589 bp of the 5′-upstream sequence of the cyc07 gene could direct specifical expression of the GUS reporter gene in meristematic tissues in transgenic plants. The spatial pattern of expression directed by the promoter was closely correlated with meristematic activity and cell proliferation, suggesting an association between the function of the cyc07 gene and cell proliferation.
Similar content being viewed by others
References
Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep, in press (1993).
Amino S, Fujimura T, Komamine A: Synchrony induced by double phosphate starvation in a suspension culture of Catharanthus roseus, Physiol Plant 59: 393–396 (1983).
Atanassova R, Chaubet N, Gigot C: A 126 bp fragment of a plant histone gene promoter confers preferential expression in meristems of transgenic Arabidopsis. Plant Cell 2: 291–300 (1992).
Brown SC, Bergounioux C, Tallet S, Marie D: Flow cytometry of nuclei for ploidy and cell cycle analysis. In: Negrutiu I, Gharti-Chhetri G (eds) A Laboratory Guide for Cellular and Molecular Plant Biology, pp. 326–345. Birkhauser, Basel (1991).
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, Bi FY: Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18: 659–668 (1975).
Colasanti J, Tyers M, Sundaresan V: Isolation and characterization of cDNA clones encoding a functional p34cdc2 homologue from Zea mays. Proc Natl Acad Sci USA 88: 3377–3381 (1991).
Doonan JH: Cycling plant cells. Plant J 1: 129–132 (1991).
Feiler HS, Jacobs TW: Cell division in higher plants: a cdc2 gene, its 34-kDa product, and histone H1 kinase activity in pea. Proc Natl Acad Sci USA 87: 5397–5401 (1990).
Ferreira PCG, Hemerly AS, Villarroel R, Van Montagu M, Inze D: The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell 3: 531–540 (1991).
Fujimura T, Komamine A: Effects of various growth regulators on the embryogenesis in a carrot suspension culture. Plant Sci Lett 5: 359–364 (1975).
Garrett JM, Singh KK, Vonder Haar RA, Emr SD: Mitochondrial protein import: isolation and characterization of the Saccharomyces cerevisiae MFT1 gene. Mol Gen Genet 225: 483–491 (1991).
Gigot C: Histone genes in higher plant. In Kahl G (ed) Architecture of Eukaryotic Genes, pp. 1367–1373, VCH Verlagsgesellschaft, Weinheim (1988).
Hata S, Kouchi H, Suzuka I, Ishii T: Isolation and characterization of cDNA clone for plant cyclin. EMBO J 10: 2681–2688 (1991).
Hemerly A, Bergounioux C, van Montagu M, Inze D, Ferreira P: Genes regulating the plant cell cycle: isolation of a mitotic-like cyclin form Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 3295–3299 (1992).
Hirt H, Pay A, Gyorgyey J, Bako L, Nemeth K, Bogre L, Schweyen RJ, Heberle-Bors E, Dudits D: Complementation of a yeast cell cycle mutant by alfalfa cDNA encoding a protein kinase homologous to p34cdc2. Proc Natl Acad Sci USA 88: 1636–1640 (1991).
Holsters M, de Waele D, Depicker A, Messens E, Van Montagu M, Schell J: Transfection and transformation of A. tumefaciens. Mol Gen Genet 163: 181–187 (1978).
Ito M, Kodama H, Komamine A: Identification of a novel S-phase-specific gene during the cell cycle in synchronous cultures of Catharanthus roseus cells. Plant J 1: 141–148 (1991).
Ito M, Yasui A, Komamine A: A gene family homologous to the S-phase specific gene in higher plants is essential for cell proliferation in Saccharomyces cerevisiae. FEBS Lett 301: 29–33 (1992).
Ito M, Yasui A, Komamine A: Precise mapping and molecular characterization of the MFT1 gene involved in import of a fusion protein into mitochondria in Saccharomyces cerevisiae. FEBS Lett 320: 125–129 (1993).
John PCL, Sek FJ, Lee MG: A homolog of the cell cycle control protein p34cdc2 participates in the division cycle of Chlamydomonas, and a similar protein is detectable in higher plants and remote taxa. Plant Cell 1: 1185–1193 (1989).
Kato K, Shiozawa Y, Yamada A, Nishida K, Noguchim M: A jar fermentation culture of Nicotiana tabacum L. suspensions. Agric Biol Chem 36: 899–902 (1972).
Kho C-J, Zarbl H: Fte-1, a v-fos transformation effector gene, encodes the mammalian homologue of a yeast gene involved in protein import into mitochondria. Proc Natl Acad Sci USA 89: 2200–2204 (1992).
Kodama H, Ito M, Hattori T, Nakamura K, Komamine A: Isolation of genes that are preferentially expressed at the G1/S boundary during the cell cycle in synchronized cultures of Catharanthus roseus cells. Plant Physiol 95: 406–411 (1991).
Kodama H, Ito M, Ohishi N, Suzuka I, Komamine A: Molecular cloning of the gene for plant proliferating-cell nuclear antigen and expression of this gene during the cell cycle in synchronized cultures of Catharanthus roseus cells. Eur J Biochem 197: 495–503 (1991).
Kodama H, Kawakami N, Watanabe A, Komamine A: Phase-specific polypeptides and poly(A)+ RNAs during the cell cycle in synchronous cultures of Catharanthus roseus cells. Plant Physiol 89: 910–917 (1989).
Koning AJ, Tanimoto EY, Kiehne K, Rost T, Comai L: Cell-specific expression of plant histone H2A genes. Plant Cell 3: 657–665 (1991).
Kosugi S, Suzuka I, Ohashi Y, Murakami T, Arai Y: Upstream sequences of rice proliferating cell nuclear antigen (PCNA) gene mediate expression of PCNA-GUS chimeric gene in meristems of transgenic tobacco plants. Nucl Acids Res 19: 1571–1576 (1991).
Lehrach H, Diamond D, Wozney JM, Boedtker H: RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16: 4743–4751 (1977).
Lepetit M, Ehling M, Chaubet N, Gigot C: A plant histone gene promoter can direct both replication-dependent and-independent gene expression in transgenic plants. Mol Gen Genet 231: 276–285 (1992).
Martinez MC, Jorgensen J-E, Lawton MA, Lamb CJ, Doerner PW: Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc Natl Acad Sci USA 89: 7360–7364 (1992).
McKinney JD, Heintz N: Transcriptional regulation in the eukaryotic cell cycle. Trends Biochem Sci 16: 430–435 (1991).
Medford JI: Vegetative apical meristems. Plant Cell 4: 1029–1039 (1991).
Medford JI, Elmer JS, Klee HJ: Molecular cloning and characterization of genes expressed in shoot apical meristems. Plant Cell 3: 359–370 (1991).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Murray MG, Thompson WF: Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8: 4321–4325 (1980).
Nishida T, Ohnishi N, Kodama H, Komamine A: Establishment of synchrony by starvation and readdition of auxin in suspension cultures of Catharanthus roseus cells. Plant Cell, Tissue Organ Culture 28: 37–43 (1992).
Nurse P: Universal control mechanism regulating onset of M-phase. Nature 344: 503–508 (1990).
Ohta S, Mita S, Hattori T, Nakamura K: Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31: 805–813 (1990).
Okada K, Takebe I, Nagata T: Expression and integration of genes introduced into highly synchronized plant protoplasts. Mol Gen Genet 205: 398–403 (1986).
Ozawa K, Komamine A: Establishment of a system of high-frequency embryogenesis from long-term cell suspension cultures of rice (Oryza sativa L.). Theor Appl Genet 77: 205–211 (1989).
Pri-Hadash A, Hareven D, Lifschitz E: A meristem-related gene from tomato encodes a dUTPase: Analysis of expression in vegetative and floral meristems. Plant Cell 4: 149–159 (1992).
Radke SE, Andrews BM, Moloney MM, Crouch ML, Kridl JC, Kmauf VC: Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75: 685–694 (1988).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schmidt GW, Bartlett SG, Grossman AR, Cashmore AR, Chua N: Biosynthetic pathway of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol 91: 468–478 (1981).
Smith JA, Krauss MR, Borkird C, Sung ZR: A nuclear protein associated with cell divisions in plants. Planta 174: 462–472 (1988).
Suzuka I, Daidoji H, Matsuoka M, Kadowaki K, Takasaki Y, Nakane PK, Moriuchi T: Gene for proliferating-cell nuclear antigen (DNA polymerase δ auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci USA 86: 3189–3193 (1989).
Suzuka I, Hata S, Matsuoka M, Kosugi S, Hashimoto J: Highly conserved structure of proliferating cell nuclear antigen (DNA polymerase δ auxiliary protein) gene in plants. Eur J Biochem 195: 571–575 (1991).
Tanaka A, Mita S, Ohta S, Kyozuka J, Shimamoto K, Nakamura K: Enhancement of foreign gene expression by a dicot intron in rice but not in tobacco is correlated with an increased level of mRNA and efficient splicing of the intron. Nucl Acids Res 18: 6767–6770 (1990).
Uematsu C, Murase M, Ichikawa H, Imamura J: Agrobacterium-mediated transformation and regeneration of kiwi fruit. Plant Cell Rep 10: 286–290 (1991).
Ursin VM, Irvine JM, Hiatt WR, Shewmaker CK: Developmental analysis of elongation factor-1α expression in transgenic tobacco. Plant Cell 3: 583–591 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ito, M., Sato, T., Fukuda, H. et al. Meristem-specific gene expression directed by the promoter of the S-phase-specific gene, cyc07, in transgenic Arabidopsis . Plant Mol Biol 24, 863–878 (1994). https://doi.org/10.1007/BF00014441
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00014441