Abstract
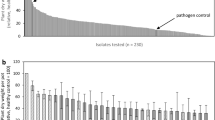
Selected bacterial strains isolated from the region of peanut pod development (geocarposphere) and two additional bacterial strains were screened as potential biological control agents against Aspergillus flavus invasion and subsequent aflatoxin contamination of peanut in laboratory, greenhouse, and field trials. All 17 geocarposphere strains tested delayed invasion of young roots and reduced colonization by the fungus in a root-radicle assay used as a rapid laboratory prescreen. In a greenhouse study, seven bacterial strains significantly reduced pod colonization by A. flavus compared to the control. In a field trial, conducted similarly to the greenhouse assay, pods sampled at mid-peg from plants seed-treated with suspensions of either 91A-539 or 91A-550 were not colonized by A. flavus, and the incidence of pods invaded from plants treated with either 91A-539 or 91A-599 was consistently lower than nonbacterized plants at each of five sampling dates. At harvest, 8 geocarposphere bacterial strains significantly lowered the percentage of pods colonized (> 51%) compared to the control. Levels of seed colonization ranged from 1.3% to 45% and did not appear related to aflatoxin concentrations in the kernels.
Similar content being viewed by others
References
Blankenship P D, Cole R J and Sanders T H 1985 Comparative susceptibility of four experimental peanut lines and the cultivar Florunner to preharvest aflatoxin contamination. Peanut Sci. 12, 70–72.
Bowen K L, Kloepper J W, Chourasia H and Mickler C J 1992 Selection of geocarposphere bacteria as candidate biological control agents for aflatoxigenic fungi and reducing aflatoxin contamination in peanut. (Abstr.) Phytopathology 82, 1121.
Brenneman T B, Wilson D M and Beaver R W 1993 Effects of diniconazole on Aspergillus populations and aflatoxin formation in peanut under irrigated and nonirrigated conditions. Plant Dis. 77, 608–612.
Brown R L, Cotty P J and Cleveland T E 1991 Reduction in aflatoxin content of maize by atoxigenic strains of Aspergillus flavus. J. Food Prot. 54, 623–626.
Cole R J, Csinos A S, Blankenship P D, Sanders T H, Gaines T P and Davidson J I 1985a Evaluation of soil calcium as methods of prevention of preharvest aflatoxin contamination of peanuts. (Abstr.) Proc. Am. Peanut Res. Educ. Soc. 17, 71.
Cole R J, Sanders T H, Hill R A and Blankenship P D 1985b Mean geocarposphere temperatures that induce preharvest aflatoxin contamination of peanuts under drought stress. Mycopathologia 91, 41–46.
Cook R J and Baker K F 1983 The Nature and Practice of Biological Control of Plant Pathogens. APS Press, St. Paul, MN. 539p.
Cotty P J 1990 Effect of atoxigenic strains of Aspergillus flavus on aflatoxin contamination of developing cottonseed. Plant Dis. 74, 233–235.
Dorner J W, Cole R J and Blankenship P D 1992 Use of a biocompetitive agent to control preharvest aflatoxin in drought stressed peanuts. J. Food Prot. 55, 888–892.
Fravel D R 1988 Role of antibiosis in the biocontrol of plant diseases. Annu. Rev. Phytopathol. 26, 75–91.
Garren K H 1966 Peanut (groundnut) microfloras and pathogenesis in peanut pod rot. Phytopathol. Z. 55, 359–367.
Griffin G J and Garren K H 1974 Population levels of Aspergillus flavus and the A. niger group in Virginia peanut field soils. Phytopathology 64, 322–325.
Kloepper J W 1991 Development of in vivo assays for prescreening antagonists of Rhizoctonia solani on cotton. Phytopathology 81, 1006–1013.
Kloepper J W and Bowen K L 1991 Quantification of the geocarposphere and rhizosphere effect of peanut (Arachis hypogaea L.). Plant and Soil 136, 103–109.
Kloepper J W, McInroy J A and Bowen K L 1992 Comparative identification by fatty acid analysis of soil, rhizosphere, and geocarposphere bacteria of peanut (Arachis hypogaea L.). Plant and Soil 139, 85–90.
Onkau D D and Sinclair J B 1985 Basic Plant Pathology Methods. CRC Press, Boca Raton, FL. 355p.
Pettit R E and Taber R A 1968 Factors influencing aflatoxin accumulation in peanut kernels and the associated mycoflora. Appl. Microbiol. 16, 1230–1234.
Pettit R E, Taber R A, Schroeder H W and Harrison A L 1971 Influence of fungicides and irrigation practice on aflatoxin in peanuts before digging. Appl. Microbiol. 22, 629–634.
SAS Institute 1985 SAS User's Guide: Statistics. Version 5 ed SAS Institute, Cary, NC.
Weller D M 1988 Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26, 379–407.
Wilson D M and Stansell J R 1983 Effect of irrigation regimes on aflatoxin contamination of peanut pods. Peanut Sci. 10, 54–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mickler, C.J., Bowen, K.L. & Kloepper, J.W. Evaluation of selected geocarposphere bacteria for biological control of Aspergillus flavus in peanut. Plant Soil 175, 291–299 (1995). https://doi.org/10.1007/BF00011365
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00011365