Abstract
Nanotechnology has been a key area of funding and policy for the United States and globally for the past two decades. Since nanotechnology research and development became a focus and nanoproducts began to permeate the market, scholars and scientists have been concerned about how to assess the risks that they may pose to human health and the environment. The newest generation of nanomaterials includes biomolecules that can respond to and influence their environments, and there is a need to explore whether and how existing risk-analysis frameworks are challenged by such novelty. To fill this niche, we used a modified approach of upstream oversight assessment (UOA), a subset of anticipatory governance. We first selected case studies of “active nanomaterials,” that are early in research and development and designed for use in multiple sectors, and then considered them under several, key risk-analysis frameworks. We found two ways in which the cases challenge the frameworks. The first category relates to how to assess risk under a narrow framing of the term (direct health and environmental harm), and the second involves the definition of what constitutes a “risk” worthy of assessment and consideration in decision making. In light of these challenges, we propose some changes for risk analysis in the face of active nanostructures in order to improve risk governance.




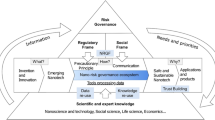
(Adapted from Renn 1992)

(Adapted from Zhang et al. 2014)

(Adapted from Grove et al. 2013)

(Adapted from Brown et al. 2013)

(Adapted from Brown et al. 2013)

Similar content being viewed by others
References
Anderson M et al (2004) Risk assessment for invasive species. Risk Anal 24(4):787–793
Arts JHE et al (2015) A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul Toxicol Pharmacol 71(2):S1–S27
Barben D, Fisher E, Selin C, Guston DH (2008) Anticipatory governance of nanotechnology: foresight, engagement, and integration. In: Hackett EJ, Amsterdamska O, Lynch ME, Wajcman J (eds) The New Handbook of Science and Technology Studies. MIT Press, Cambridge, pp 979–1000
Blatch GL, Lässle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932–939
Brown PK, Qureshi AT, Moll AN, Hayes DJ, Monroe WT (2013) Silver nanoscale antisense drug delivery system for photoactivated gene silencing. ACS Nano 7(4):2948–2959
Byrd DM, Cothern CR (2005) Introduction to risk analysis: a systematic approach to science-based decision making. Ecological risk analysis. Government Institutes Press, Lanham, pp 311–327
Castellano L, Stebbing J (2013) Deep sequencing of small RNAs identifies canonical and non-canonical miRNA and endogenous siRNAs in mammalian somatic tissues. Nucleic Acids Res 41:3339–3351
EPA (1998) Guidelines for ecological risk assessment. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, EPA/630/R095/002F
Ezell BC et al (2010) Probabilistic risk assessment and terrorism risk. Risk Anal 30(4):575–589
FDA (2015a) Food and drug administration. Biotechnology consultation—note to file biotechnology notification file no. 132. http://www.fda.gov/Food/FoodScienceResearch/Biotechnology/Submissions/ucm436168.htm
FDA (2015b) Food and drug administration. Biotechnology consultation—note to file biotechnology notification file no. 141. http://www.fda.gov/Food/FoodScienceResearch/Biotechnology/Submissions/ucm436173.htm
Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K et al (2006) Off-target effects by siRNA can induce toxic phenotype. RNA 12(7):1188–1196
Fjeld R et al (2007) Chapter 11. Dose-response and risk characterization. Quantitative environmental risk analysis for human health. Wiley, Hoboken, pp 245–282
Funtowicz, S, Ravetz J (2008) Post-normal science. In: Cleveland CJ (ed) Encyclopedia of earth. Environmental Information Coalition, National Council for Science and the Environment, Washington, DC
Gavankar S et al (2012) Life cycle assessment at nanoscale: review and recommendations. Int J Life Cycle Assess 17:295–303
Grimm D (2011) The dose can make the poison: lessons learned from adverse in vivo toxicities caused by RNAi overexpression. Silence 2(1):1–6
Grove TZ, Forster J, Pimienta G, Dufresne E, Regan L (2012) A modular approach to the design of protein-based smart gels. Biopolymers 97(7):508–517
Grove TZ, Regan L, Cortajarena AL (2013) Nanostructured functional films from engineered repeat proteins. J R Soc Interface 10(83):20130051
Gu HZ, Chao J, Xiao SJ, Seeman NC (2010) A proximity-based programmable DNA nanoscale assembly line. Nature 465:202–205
Guston DH (2014) Understanding ‘anticipatory governance’. Soc Stud Sci 44(2):218–242
Guston D, Sarewitz D (2002) Real-time technology assessment. Technol Soc 23:93–109
Helwak A, Kudla G, Dudnakova T, Tollervey D (2013) Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153:654–665
IRGC (2006) White paper on risk governance: towards an integrative approach. International Council on Risk Governance, Geneva. http://www.irgc.org
IRGC (2007) Nanotechnology risk governance. Policy brief. International Risk Governance Council, Geneva. http://www.irgc.org
Katoch R, Sethi A, Thakur N, Murdock LL (2013) RNAi for insect control: current perspective and future challenges. Appl Biochem Biotechnol 171(4):847–873
Kaushik R, Balasubramanian R (2014) A comparative toxicity evaluation of Escherichia coli-targeted ssDNA and chlorine in HepG2 cells. Water Res 48:519–528
Kuzma J (2010) Nanotechnology in animal production: upstream assessment of applications. Livest Sci 130:14–24
Kuzma J, Besley J (2008) Ethics of risk analysis and regulatory review: From bio- to nanotechnology. Nanoethics 2(2):149–162
Kuzma J, Verhage P (2006) Nanotechnology in agriculture and food production. Project on emerging nanotechnologies PEN4. Woodrow, Washington, DC
Kuzma J, Romanchek J, Kokotovich A (2008) Upstream oversight assessment for agrifood nanotechnology. Risk Anal 28:1081–1098
Lin C, Rinker S, Wang X, Liu Y, Seeman NC, Yan H (2008) In vivo cloning of artificial DNA nanostructures. Proc Natl Acad Sci 105(46):17626–17631
Lin C, Liu Y, Yan H (2009) Designer DNA nanoarchitectures. Biochemistry 48(8):1663–1674
MacPhail RC et al (2013) Assessing nanoparticle risk poses prodigious challenges. WIREs Nanomed Nanobiotechnol 5:374–387
Maynard A et al (2011) The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol Sci 120(S1):S109–S129
Miller G, Wickson F (2015) Risk analysis of nanomaterials: exposing nanotechnology’s naked emperor. Rev Policy Res 32(4):485–512
NRC (1983) Risk assessment in the federal government: managing the process. National Academies Press, Washington, DC
NRC (1996) Understanding risk. National Academies Press, Washington, DC
NRC (2009) Science and decisions. National Academies Press, Washington, DC
NSF (2006) Active nanostrutures and nanosystems (ANN). National Science Foundation Solicitation, NSF 06-595
Olsen SI et al (2001) Life cycle impact assessment and risk assessment of chemicals—a methodological comparison. Environ Impact Assess Rev 21(4):385–404
Paradise J, Tisdale AW, Hall RF, Kokkoli E (2009) Evaluating oversight of human drugs and medical devices: a case study of the FDA and implications for nanobiotechnology. J Law Med Ethics 37(4):598–624
Parkin R (2007) Microbial risk assessment. Chapter 11. In: Robson MG, Toscano WA (eds) Risk assessment for environmental health. Wiley, San Francisco, pp 285–313
Petrick JS, Brower-Toland B, Jackson AL, Kier LD (2013) Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: a scientific review. Regul Toxicol Pharmacol 66(2):167–176
Renn O (1992) Concepts of risk: a classification. Chapter 3. In: Krimsky S (ed) Social theories of risk. Praeger, Westport, pp 53–79
Roco M (2004) Nanoscale science and engineering: unifying and transforming tools. AIChE J 50:890–897
Selness AR, Vennette RC (2006) Minnesota pest risk assessment: Emerald Ash Borer. MN Department of Agriculture Publication number: PRA-APLA-001
Sharp P (2001) RNA interference—2001. Genes Dev 15:485–490
Shatkin JA (2008) Informing environmental decision making by combining life cycle assessment and risk analysis. J Ind Ecol 12(3):278–281
Shi Y, Zhang J, Jiang M, Zhu L, Tan H, Lu B (2010) Synergistic genotoxicity caused by low concentration of titanium dioxide nanoparticles and p,p′-DDT in human hepatocytes. Environ Mol Mutagen 51(3):192–204
Stirling A (2007) Risk, precaution and science: towards a more constructive policy debate. EMBO Rep 8(4):309–315
Subramanian V, Youtie J et al (2010) Is there a shift to “active nanostructures”? J Nanopart Res 12(1):1–10
USDA (2014) Okanagan specialty fruits Inc.’s petition (10-161-01p) for determination of non-regulated status of non-browning arcticTM apple events GD743 and GS784. https://www.aphis.usda.gov/brs/aphisdocs/10_16101p_dea.pdf
Wang T et al (2011) Self-replication of information-bearing nanoscale patterns. Nature 478:225–228
Yang J, Hirschi KD, Farmer L (2015) Dietary RNAs: New stories regarding oral delivery. Nutrients 7:3184–3199
Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S et al (2013) Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci 20:4–14
Zhang X, Zhang J, Zhu KY (2010) Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 19:683–693
Zhang L et al (2012) Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 22:107–126
Zhang F et al (2014) Structural DNA nanotechnology: state of the art and future perspective. J Am Chem Soc 136:11198–11211
Acknowledgments
This work was supported by the National Science Foundation (NSF) Award to the Center for Nanotechnology in Society at ASU (Guston) and Ndnano at Notre Dame (Eggleson) #1235693 and by the Genetic Engineering and Society Center at North Carolina State University (www.research.ncsu.edu/ges). The findings and observations contained in this article are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Kathleen Eggleson, David H. Guston
This article is part of the Special Focus on Anticipatory Governance of Next Generation Nanotechnology
Rights and permissions
About this article
Cite this article
Kuzma, J., Roberts, J.P. Is adaptation or transformation needed? Active nanomaterials and risk analysis. J Nanopart Res 18, 215 (2016). https://doi.org/10.1007/s11051-016-3506-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3506-y