Abstract
Bio-degradable polymers have attracted much attention and been familiar to be the most promising drug carrier materials for the last several years due to their wonderful virtues such as easily existing, non-toxic, bio-compatible, and can be modified easily. Herein, we report the synthesis of new drug carrier poly (acrylamide-co-diallyldimethylammoniumchloride) nanogels (PAD-NG) by dispersion polymerization technique using free radical initiator potassium persulphate (KPS), potassium hydrogen sulphate (KHSO4) as a co-initiator and chemical cross-linker N, N-methylene bisacrylamide (NN-MBA). The developed nanogels are characterized for their structure, size and morphology. The average size of the nanogels was about 100 nm. These nanogels were used as intracellular drug delivery vehicle by encapsulating 5-fluorouracil (5-FU) with efficiency of 76.34%. Further, placebo nanogels showed negligible hemolytic activity and cytotoxicity against neuroblastoma cells.
Similar content being viewed by others
1 Introduction
Polymer nanotechnology has opened up extraordinary opportunities to fabricate controlled drug release devices, which has resulted several advantages to deliver small molecules like pharmaceutical agents. The preparation and design of hydrogels have attracted a great deal of interest in biomedical engineering and pharmaceutical applications because of their tunable chemical and three-dimensional (3-D) structure, high loading aqueous content and excellent biocompatibility [1]. Hydrogels with nanosize range are called as nanogels (NGs). Recently, the development of nanosized gel 3-D structures in sub-micron size range from physically or chemically cross-linked polymer networks have much attention due to their special features such as good mechanical properties, small size, large surface area, colloidal stability, and high drug loading capacity showing the characteristics of both hydrogels as well as nanomaterials [2, 3]. The size controllable feature of NGs along with the uniformly cross-linked matrix adds further advantage to fabrication, to utilize as carrier in the development of burst or prolonged controlled release drug delivery systems [4]. With large surface area for multivalent bio-conjugation and interior network for the incorporation of bio-molecules make them more advantageous than micelles, vesicles, liposomes, etc. for biomedical applications [5,6,7].
Nanogels have been developed by different ways such as physical self assembly, homo or heterogeneous polymerization of monomers, cross-linking of preformed polymers, microfluidics, micromolding, photolithography, precipitation polymerization and inverse emulsion polymerization [8,9,10]. For the last few years, many reports have been published on the development of biocompatible NGs using different monomeric units [11,12,13]. Ashwanikumar et al. [11] reported methacrylic based NGs for the pH-sensitive 5-FU drug delivery in the colon. Cell proliferation assay of cancer cell lines (HCT-116) was investigated and stated that the NGs containing 5-FU exhibited significant cytotoxicity compared with free 5-FU. Antonie et al. [12] reported surfactant free highly stabilized NGs of zwitterionic poly (amido amine) with minimal cytotoxicity were treated with three cell lines. Now-a-days, research in polymer based nanocarriers is focused on development of new multi structural polymer devices because of their better performance than that of individual polymers and the main advantage of introducing functional groups of interest, which can later be utilized for the binding of targeting sites, bio-molecules or for the covalent conjugation of chemotherapeutic agents [13, 14]. Different co-polymers have been utilized for the development of stimuli-responsive NGs that can release the incorporated drug in response to local physiological conditions like pH, ionic strength, temperature and also redox environment [15]. Alvarez-Bautista et al. [16] developed nano-hydrogels of different composition by microemulsion polymerization using N-isopropylacrylamide as a base monomer and 1-vinyl imidazole as ionizable co monomer. The developed NGs were functionalized with folic acid to take advantage that the folate receptor is over expressed in different types of cancer cells. Naga Sravan Kumar et al. [17] developed poly (N-isopropylacrylamide-co-acrylic acid) (PNA) NGs via free radical polymerization. In this study, in vitro drug release of 5-FU, percentage of hemolysis, pH dependent cytotoxicity, in vivo kinetics on Wistar rats and in vivo anti-cancer efficacy and means life span on Swiss albino mice were investigated. Dolores Blanco et al. [18] developed submicron size hydrogel composed of N-isopropylacrylamide for 5-FU drug delivery and investigated the cyotoxic studies on MCF7 and HeLa cells. NGs are mostly spherical shaped particles but the current advancement in synthetic strategies allow for the fabrication of different shapes. They have been used for the delivery of various components such as drugs, imaging labels, proteins, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), genes and growth factors [19, 20].
A variety of nanocarriers have been used to encapsulate 5-FU in order to promote long time release of the drug and improve accumulation concentration in the body, thereby improving the antitumor efficacy [21, 22]. 5-FU is a typical pyrimidine analogue, anti tumar agent, widely used in the healing of a wide range of solid malignant tumors and broadly used in cancer chemotherapy [23]. It is partially water soluble, hydrophilic acidic in nature and also an antineoplastic agent of widely used in clinical chemotherapy for the action of solid tumors [22].
Diallyldimethylammoniumchloride (DADMAC) is a synthetic highly water soluble quaternary ammonium monomer and its homo and copolymers [polyelectrolytes] are generally used in water treatment, mining, paper manufacturing, cosmetics, biology and drug delivery applications [24, 25] and also as depressor of fungi and bacteria because of its quaternary nature [26]. The charge density and molecular weight are two key parameters which influence properties and end use applications of these polymers. Use of such polyelectrolytes for controlled release applications is limited and in addition these materials are less toxic and relatively cheap. This prompted us to develop controlled release formulations using these polyelectrolytes.
In the present study, the synthesis and characterization of poly (acrylamide-co-diallyldimethylammoniumchloride) NGs has been reported via free radical dispersion polymerization as a delivery vehicle for 5-FU. Finally, the blood compatability and cytotoxicity investigation was performed on SK-N-SH cell line (human neuroblastoma cells) in order to assess the efficacy of the formulation.
2 Experimental methods
2.1 Materials
Poly(vinyl pyrrolidone) (PVP) average Mw 360,000, acrylamide (Am), diallyldimethylammoniumchloride (DADMAC) 65 wt% solution in water, methanol and triton-x, MTT (3-(4, 5-dimethylthiozol-2-yl)-2, 5-diphenyltetrazoliumbromide) were purchased from Sigma Aldrich, USA. N, N- methylene bisacrylamide (NN-MBA), potassium hydrogen sulphate (KHSO4) and potassium persulphate (KPS) were received from s. d. fine chemicals, Mumbai, India and 5-fluorouracil (5-FU) from Himedia Chemicals, India. Double distilled water collected from equipment Distillon was used throughout our study.
2.2 Synthesis of poly (Am-co-DADMAC) NGs
Poly (Am-co-DADMAC) (PAD-NG) nanogels were prepared by using dispersion polymerization technique using our previously published protocol with further modifications [27]. In brief, in a four neck round bottom flask, required amount of PVP was dissolved in 100 mL of methanol–water solvent mixture (1:1) v/v ratio. Mechanical stirrer, reflux condenser, a nitrogen gas inlet and dropping funnel were fixed to four necks of the flask. Nitrogen gas was purged throughout the reaction. Both, DADMAC and acrylamide monomers and cross-linker NN-MBA were dissolved in water and added to the flask through dropping funnel and the temperature of the reaction mixture was raised to 70 °C using oil bath. Once the temperature was obtained, both initiator and co-initiator dissolved in water was added drop-wise slowly over a period of 40 min through dropping funnel. During first 1 h, turbidity was observed in the reaction mixture, which is the indication of the formation of poly (Am-co-DADMAC). However, the reaction was continued for 8 h for maximum conversion. After that the reaction mixture was cooled and the NGs were collected by centrifuging at about 20,000 rpm (approx 50,000 g) for 45–60 min. The sediment was washed 2–3 times with distilled water to eliminate un-reacted monomers and PVP from the NGs. Drug (5-FU) loaded NGs were prepared in the same manner by adding the drug along with monomers. To determine the effect of NN-MBA cross-linker and 5-FU loading on release rate from the NGs, a total of five types of drug-loaded NGs were prepared by varying the amount of drug at a constant NN-MBA content, and amount of NN-MBA at a constant drug amount and the details are given in Table 1.
2.3 Characterization of nanogels
Infrared spectra was recorded using FTIR spectrophotometer (Perkin Elmer, model Two, UK) of pure 5-FU, placebo and 5-FU loaded NGs. The samples were finely grinded with KBr and pellet was made under hydraulic pressure of 600 dyn/m2 and spectra were recorded in the range of 4000–400 cm−1. X-ray diffraction (XRD) patterns of pure 5-FU, placebo and drug loaded NGs were recorded on RIGAKU SMART LAB mini 600 (Japan) using Cu Kα radiation X-ray diffractometer to find out the crystallinity of the samples. The XRD scans were performed from 10° to 60° of 2 theta values. Differential scanning calorimetry (DSC) analysis of pure 5-FU drug, placebo and drug loaded NGs were performed on a Rheometric scientific STA, TG instruments, SDT Q600, (UK). About 10 mg of samples were used and thermo grams were recorded from 30 to 500 °C at controlled heating rate of 10° C/min under inert N2 gas. Samples for high resolution transmission electron microscopy (HR-TEM) were made by dispersing NGs in ethyl alcohol with a concentration of 10 mg/mL and dropped on 200 mesh carbon coated copper grid and placed at ambient conditions for drying. HR-TEM images were taken on a JEOL 3010 (Japan) at 20 kV microscopy. The size and distribution of the NGs was measured using Zeta sizer, Nano S 90, Malvern (UK) by dispersing the NGs in distilled water at the amount of 1 mg/mL.
2.4 Encapsulation efficiency
The drug 5-FU present in the drug encapsulated NGs was estimated by placing a specific amount of NGs in 10 mL of a pH 7.4 aqueous buffer solution and stirring continuously for a day. The dispersed solution was centrifuged and the amount of 5-FU in the supernatant liquid was analyzed by UV–Visible spectrophotometer (Lab India, Mumbai, India) measuring at fixed value of 270 nm. The percentage of drug loading (% DL) and encapsulation efficiency (% EE) were estimated using Eqs. (1) and (2), respectively.
2.5 In vitro drug release studies
To know the drug release behavior from NGs, in vitro release studies were done with tablet dissolution tester apparatus (LABINDIA, DS 8000 model), containing eight baskets. Each 5-FU loaded NG formulation was taken in dialysis bag and put in an individual basket containing buffer solutions with pH 7.4. Dissolution studies were carried out at constant speed of 100 rpm and 37 ± 0.5 °C temperature. At predetermined time intervals, 5 mL of drug released buffer were taken out from the baskets and the equal amount of fresh buffer solution was added. The samples were analyzed using UV-Visible spectrophotometer at 270 nm to estimate the amount of drug released from NGs to external buffer.
2.6 Hemocompatibility assay
The investigation of the in vitro hemolytic activity is essential to ensure blood compatibility of NGs in the case of intravenous drug delivery carriers. Hemolysis assay results in lysis of the red blood cells by damage of the cell membrane which will lead to adverse effects, and may cause hemolytic anemia [28]. About 20 mL of fresh human blood was collected in tubes coated with heparin to prevent blood coagulation. Then blood was centrifuged at 500×g for 5 min, three different layers were noticed red lower layer (Red Blood Cells), white buffy coat (White Blood Cells) and plasma upper layer. Aspirate plasma and white buffy coat with micropipette and fill the blood tubes to marked line with 150 mM NaCl solution and then invert the tubes for few minutes to mix the solution. Centrifuge tubes at 500×g for 5 min to separate the solution. Repeat steps to wash blood cells again and aspirate supernatant and replace with 0.1 M PBS at pH 7.4 invert to mix. Invert to mix properly and proceed tored blood cells lysis assay in 96 well plate. Equal volume of RBC cells (190 μL) was added to wells and then added 20 μL of 20% triton-x 100 for positive control wells, and for negative control wells added phosphate buffer saline and 20 μL of NGs of different concentration were added for test wells. Then plates were incubated at 37 °C for 1 h. Later centrifuged the plates for 5 min. at 500×g to pellet intact RBCs. Without disturbing the pellet using a multichannel pipette, transfered 100 μL of supernatant from each well into another clear flat bottom 96 well plate. Absorbance of supernatants was read at 490 nm with a plate reader. Experiments were repeated three times and the results represented as averages with standard error.
2.7 Cell culture and cytotoxicity measurement by (MTT) assay
SK-N-SH cell line (human neuroblastoma cells) was obtained from National Centre for Cell Sciences, Pune, India. SK-N-SH was cultured in MEM containing 0.5 mM l-glutamine, 0.1 mM sodium pyruvate and 1 mM non-essential amino acids with 10% FBS. Cells were maintained at 37 °C and 5% CO2. Cell cultures at approximately 80% confluence were used for all in vitro experimental procedures. In this work, cytotoxic effect of prepared placebo, drug loaded NGs and 5-FU on the cell viability was measured by using MTT assay as described in previous studies [29]. SK-N-SH cells (0.2 × 106 cells per well) in 200 μL of corresponding medium with 10% FBS were seeded into 96-well plate. Increasing concentrations of NGs were added to the cells and incubated at 37 °C for 24 h in a humidified CO2 incubator with 5% CO2. The medium was replaced along with 20 μL of 5 mg/mL MTT (3-(4, 5-dimethylthiozol-2-yl)-2, 5-diphenyltetrazoliumbromide). It was further incubated for 4 h in humidified atmosphere, the medium was removed and 200 μL of 0.1 N acidic isopropyl alcohol was added to the wells to dissolve the MTT formazan crystals. Absorbance was recorded at 570 nm immediately after the development of purple color. Relative cell viability was evaluated according to the quantity of MTT converted into insoluble formazan salt. The optical density of the formazan generated in the control cells was considered to represent 100% viability. The results expressed as mean percent of viable cells versus respective control. Experiments were repeated three times and the results represented as averages with standard error.
3 Results and discussion
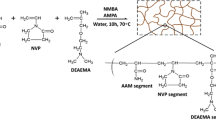
Development of new types of polymer nanocarriers is important to improve the release time, targeted delivery systems, bioavailability of the drugs and most important decreasing the cytotoxicity. In view of this, we prepared chemically, NN-MBA, cross-linked poly (Am-co-DADMAC) copolymer NGs using dispersion polymerization. A model cross-linked poly (Am-co-DADMAC) copolymers structure is presented in Fig. 1. These kinds of copolymer are widely used as flocculants and also as whitening agents in paper industry. It is expected that the unique properties of these polyelectrolytes can facilitate the improved efficacy of the NGs in controlled release applications. Dispersion polymerization was used because the formed NGs can be isolated easily from the reaction mixture and characteristics of NGs can easily be controlled by varying either methanol–water ratio or the concentration of stabilizer PVP. During this experiment, optimum design was evaluated to select the best experimental condition for preparation of NGs. This design uses the three different concentrations of NN-MBA varied from 0.05 to 0.15 wt%. Below 0.05 wt% cross-linking agent, the formations of NGs were not found during the experiment. At the same time, we have optimized the methanol and water ratio as 1:1 volume ratio. Three series of nanogels with different cross-linking agents were prepared and designated as PAD-NG.
3.1 Characterization of nanogels
FTIR spectra of the copolymer were recorded to confirm the copolymerization of acrylamide with DADMAC. FTIR spectra of (a) pure 5-FU drug, (b) placebo NGs and (c) 5-FU drug loaded NGs were shown in Fig. 2a. At high frequency region broad band between 3000 and 3500 cm−1 observed in 5-FU drug spectrum corresponds to –NH stretching vibrations [30]. The band at 3195 cm−1 illustrated the existence of N–H stretching vibrations of acrylamide of the copolymer [31]. Two sharp bands at 1659 and 1613 cm−1 are corresponds to carbonyl and N–H stretching, bending vibrations of acrylamide, respectively [32]. Bands at 1450 and 1416 cm−1 are corresponds to –CN bond stretching vibrations, which confirms the attendance of both Am and DADMAC in copolymeric NGs [33,34,35]. The band at 1326 cm−1 in pure 5-FU is assigned to C–F stretching vibrations was also observed in drug loaded NGs, however, the peak intensity is very low because of the masking of copolymer structures and also due to well distribution of drug in the NGs.
XRD difractograms are useful to study the crystallinity of the drug before and after the encapsulation of NGs. The XRD difractograms of (a) pure 5-FU drug (b) placebo NGs and (c) 5-FU drug loaded NGs are shown in Fig. 2b. The 5-FU shown characteristic intense peaks at 2 theta values of 16, 19, 21, 28, 31 and 33° exhibits its crystalline nature [36]. However, intense peaks have disappeared in the case of 5-FU encapsulated NGs, which showed a spectrum similar to that of placebo NGs. This may be due to the shielding of the drug molecules by the polymer matrices which were unable to be resolved by XRD. DSC is a powerful analytical device which reveals certain information about interactions and the changes that take place in the host guest complex. DSC profiles of (a) pure 5-FU (b) placebo NGs and (c) 5-FU loaded NGs are presented in Fig. 2c. 5-FU drug shows characteristic a melting endothermic sharp peak at 287 °C corresponding to its melting and polymorphism. In the case of placebo NGs and drug loaded NGs endotherm peak observed at below 100 °C temperature due to evaporation of moisture. Placebo NGs shows endotherm peak at ~ 275 °C. However, in the case of 5-FU loaded NGs the very small melting endotherm also appeared with less intense. This is due to the small amount of drug present on the surface of the NGs [31, 37]. These results suggest the molecular distribution of drug molecules within the NG polymer chains.
The histogram showing size and distribution of NGs presented in Fig. 2d suggest that synthesized NGs distributed between 60 and 160 nm in range. Majority of NGs were distributed in 100–120 nm range in size. Apart from size, zeta potential of the polyelectrolyte nanogels also have important role in formation of network structure as well as in the drug release studies due to the electrostatic influence of the cationic charge of the DADMAC. It has been reported that due to the use of quaternary ammonium compounds (cationoinc electrolytes) as commoners, positive zeta poetical has been obtained on the micro/nano gels [38,39,40,41]. However, in the present case the composition of the copolymer was not varied, hence, it is presumed that the effect of charge on the formation as well as drug release characteristics might be uniform in all the different nanocarriers studied and also due to the unavailability, zetal poetical was not measured. HR-TEM images of NGs shown in Fig. 2e revealed that the NGs were almost spherical in shape and dispersed well without much aggregation.
3.2 Drug loading (DL) and encapsulation efficiency (EE) of NGs
The calculated values for different formulations of NGs containing of poly (Am-co-DADMAC) are presented in Table 1. As the percent (%) of chemical cross-linker, NN-MBA, increased from 0.05 to 0.15%, the percentage of loading decreased, which in turn fall in the % EE of the NGs from 76.34 to 41.73%. It is apparent that as the cross-linking density increased, the mobility of polymer chains reduced for encapsulation of the 5-FU molecules, resulting in decrease of % EE. As expected, when the amount of 5-FU increased from 0.1 to 0.3% at a fixed amount of NN-MBA 0.1%, the percentages of DL and % EE values were increased.
3.3 In vitro drug release studies
To study the effect of drug concentration and cross-linking agent, 5- FU drug loaded NGs of poly (Am-co-DADMAC) in vitro drug release experiments were carried out in pH 7.4 phosphate buffer solutions. The effect of drug loading content in formulations PAD-NG-1, PAD-NG -2 and PAD-NG-3 from 0.1 to 0.3 wt% on release rate is presented in Fig. 3a at pH 7.4 buffer solution. The drug release profiles are similar for all the formulations studied. In the beginning 25–40% of drug released from the nanogels with in 20 min which helps to keep the therapeutic levels and later released slowly over 700 min. The percentage cumulative release of drug is increased as the amount of drug increased from 0.1 to 0.3% in PAD-NG-1 to PAD-NG-3 formulations. This is obvious because as the amount of drug present in the NGs is high the rate of release from NGs is also higher i.e. up to 90% at pH 7.4 with in 12 h. A physical adsorption of hydroxyl groups at a water-hydrophobic interface is likely to occur in polymer matrix in the buffer solution [24]. The % cumulative release of 5-FU from NGs with varying amount of cross-linking agent from 0.05 to 0.15% in formulations PAD-NG-2, PAD-NG-4 and PAD-NG-5, respectively is presented in Fig. 3b. The cumulative release of drug decreases as the amount of cross-linking agent NN-MBA increased from 0.05 to 0.15%. As the amount of cross-linking agent increases more and more rigid polymer networks will form thereby reduces swelling which in turn decreases the release of the drug from the NGs. However, at pH 7.4 solution, the aggregation because of the hydrophobic interaction may be disassembled upon the hydroxyl groups coupled to the hydrophobic chains. So the NGs showed the swelling in buffer solution, and the swelling increases with pH of the buffer solution. When compared to other drug carriers such as magnetic nanoparticles [42, 43] and microspheres which are sensitive to temperature [44, 45], nanogels have characteristic special features such as high loading capacity, tunable porosities, combining features of hydrogel and nanoparticle makes more versatile and viable platforms for controlled drug delivery. In addition, the positive charge of the cationic quaternary ammonium compound nanocarriers could interact well with the negatively charged cell membrane facilitating their cellular uptake [46,47,48,49].
3.4 In vitro release studies
The release kinetics of all the formulations of 5-FU loaded NGs was studied at 37 °C in aqueous phosphate buffer solution. The data were estimated from percentage cumulative release (Mt/M∞) with corresponds to time by placing the data into Eq. (3)
Here, Mt/M∞ denotes release of drug at time t, n is a diffusion parameter assessing the release phenomenon and k is constant corresponds to drug-polymer system. The type of release mechanism is given by the n value in equation, where n ≤ 0.5 follows Fickian diffusion pattern; while 0.5 < n < 1 follows non-Fickian trend [36]. The n values were calculated for the various formulations (Table 1) and in the present study n values were between 0.323 and 0.484 showed that the release mechanism followed Fickian diffusion behavior. The n value depends on the network structure of NGs. Correlation coefficients r, obtained values are 0.906–0.994 indicated that, the drug release from these NGs was in controlled release manner throughout the in vitro dissolution analysis.
3.5 Hemolysis assay
The study of in vitro hemolytic activity is important to ensure their blood compatibility in the case of intravenous drug delivery carriers [28]. NGs were tested for the hemocompatibility by RBC lysis assay. NGs showed good compatibility with RBC when the concentration was increased up to 1 mg/mL and very mild lyses at less than 2% was noticed when NGs was treated with the RBC and incubated for 1 h. This was very minimal effect when compared with the positive control triton-X. These results were represented in Fig. 4a indicated that developed NGs had good blood compatibility and were suitable for delivery of drugs in clinical applications.
3.6 Cytotoxicity study
A comparative cytotoxicity study of the effects of placebo NGs, 5-FU loaded NGs and pure 5-FU were presented in Fig. 4b. As shown in the figure, the placebo PAD-NGs in the test concentration range (up to 1000 μg/mL) showed no cytotoxicity on the SK-N-SH cells after 24 h incubation, while significant growth suppression of SK-N-SH cells was observed when cells were treated with drug loaded NGs and 5-FU. The drug loaded NGs showed slightly higher cytotoxicity in comparison to 5-FU over the experimental concentration range (150–1000 µg/mL) used in cells. This suggests that a lower concentration of 5-FU would bring about desired effect when encapsulated in NGs. The results suggesting that the placebos NGs have no cytotoxicity at the normal concentration and show good biocompatibility.
4 Conclusions
In summary, we have successfully developed copolymer nanogels of acrylamide and DADMAC using dispersion polymerization technique and confirmed by FTIR spectroscopy. These nanogels were shown good drug loading efficiency as high as 76% in encapsulating 5-FU drug. Further, the drug was well dispersed at molecular levels as evidenced by XRD and DSC studies. Prepared NGs were spherical in shape and are about 100 nm in size. The in vitro release profiles suggested that these NGs showed good controlled release characteristics. Hemocompatibility and cytotoxicity of the nanogels suggest that developed nanogel systems are biocompatible and are potential candidate for various biomedical applications including drug delivery.
References
Innocent JM, Ipek K, Besim B, Sophie C, Wolfgang HM (2019) Development of antimicrobial composite coatings for drug release in dental, orthopaedic and neural prostheses applications. SN Appl Sci 1(68):1–10
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Bromberg L (2005) Intelligent hydrogels for the oral delivery of chemotherapeutics. Expert Opin Drug Deliv 2(6):1003–1013
Chacko RT, Ventura J, Zhuang J, Thayumanavan S (2012) Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev 64:836–851
Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K (2008) The development of microgels/nanogels for drug delivery applications. Prog Polym Sci 33:448–477
Harada A, Kataoka K (2006) Supramolecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications. Prog Polym Sci 31:949–982
Buetuen V, Liu S, Weaver JVM, Bories-Azeau X, Cai Y, Armes SP (2006) A brief review of ‘schizophrenic’ block copolymers. React Funct Polym 66:157–165
Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed 48(30):5418–5429
Nicolas S, Jutta R (2010) Synthesis of nanogels/microgels by conventional and controlled radical cross linking copolymerization. Polym Chem 1:965–977
Harald W, Felix HR, Yuanchao L, Sergei SS, Harm-Anton K (2014) Poly [N-(2-hydroxypropyl) methacrylamide] nanogels by RAFT polymerization in inverse emulsion. Polym Chem 5:1711–1719
Ashwanikumar N, Nisha AK, Asha NS, Vinod Kumar GS (2012) Methacrylic-based nanogels for the pH-sensitive delivery of 5-Fluorouracil in the colon. Int J Nanomed 7:5769–5779
Antonie EE, Michelle MTJ, Karin R, Johan FJE, Jos MJP (2016) Surfactant-free preparation of highly stable zwitterionic poly (amido amine) nanogels with minimal cytotoxicity. Acta Biomater 30:126–134
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A (2007) Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5:1–18
Zhu K, Ye T, Liu J, Peng Z, Xu S, Lei J, Deng H, Li B (2013) Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int J Pharm 441:721–727
Chang YQ, Yun XS, Han C, Si XC, Xian ZZ, Ren XZ (2008) Thermosensitive P (NIPAAm-co- PAAc-co-HEMA) nanogels conjugated with transferrin for tumor cell targeting delivery. Nanotechnol 19:275102
Alvarez-Bautista A, Duarteb CMM, Mendizabald E, Katime I (2016) Controlled delivery of drugs through smart pH-sensitive nanohydrogels for anticancer therapies: synthesis, drug release and cellular studies. Design Monomer Polym 19:319–329
Naga Sravan KVV, Shivakumar HG, Syeda JF, Vegesna R, Farhath K (2016) pH and thermo-sensitive 5-fluorouracil loaded poly (Nipam-co-AAc) nanogels for cancer therapy. RSC Adv 6:105495–105507
Dolores BM, Sandra G, Marta B, Ana F, Cesar T, Rosa O, Issa K, Jose MT (2011) In vitro and In vivo evaluation of a folate-targeted copolymeric submicrohydrogel based on N-Isopropylacrylamide as 5-fluorouracil delivery system. Polymers 3:1107–1125
Kim C, Lee Y, Lee SH, Kim JS, Jeong JH, Park TG (2011) Self-cross linked polyethylenimine nanogels for enhanced intracellular delivery of siRNA. Macro Res 19:166–171
Marya M, Wattanaarsakit P, Narain R (2013) Cationic glyco-nanogels for epidermal growth factor receptor (EGFR) specific siRNA delivery in ovarian cancer cells. Polym Chem 4:3829–3836
Einmahl S, Zignani M, Varesio E, Heller J, Veuthey JL, Tabatabay C, Gurny R (1999) Concomitant and controlled release of dexamethasone and 5-fluorouracil from poly (ortho ester). Int J Pharm 185:189–198
Sommadossi JP, Gewirtz DA, Diasio RB, Aubert C, Cano JP, Goldman ID (1982) Rapid catabolism of 5-fluorouracil in freshly isolated rathepatocytes as analyzed by high performance liquid chromatography. J Biol Chem 257:8171–8176
Ermis D, Yuksel A (1999) Preparation of spray-dried microspheres of indomethacin and examination of the effects of coating on dissolution rates. J Microencapsul 16:315–324
Wandrey C, Hunkeler D (1999) Diallyldimethylammoniumchloride and its polymer. Adv Polym Sci 145:123–182
Yalong Z, Ling X, Min Y, Maolin Z, Jianrui W, Hongfei H (2006) Radiation synthesis of poly [(dimethyl aminoethylmethacrylate)-co-(diallyldimethylammoniumchloride)] hydrogels and its application as a carrier for notoginsenoside delivery. Eur Polym J 42:2959–2967
Lu J, Wang XD, Xiao CB (2008) Preparation and characterization of konjac glucomannan/poly (diallyldimethylammoniumchloride) antibacterial blend films. Carbo Polym 73:427–437
Sairam M, Babu VR, Naidu BVK, Aminabhavi TM (2006) Encapsulation efficiency and controlled release characteristics of cross linked polyacrylamide particles. Int J Pharm 320:131–136
Dinesh M, Roopan SM, Selvaraj CI, Arunachalam P (2017) Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, heamolytic and cytotoxic studies. J Photochem Photobiol B 16:764–771
Ramakrishna V, Preeti GK, Oruganti Setty H, Anand KK (2014) Neuroprotective effect of Emblica Officinalis extract against H2O2 induced DNA damage and repair in neuroblastoma cells. J Homeop Ayurv Med S1(002):1–5
Nada AA, Alkady M, Fekry H (2007) Synthesis and characterization of grafted cellulose for use in water metal ion sorption. BioResources 3:46–59
Periyakaruppan P, Anbalagan S, Mariappan R (2019) Synthesis of bio-degradable poly (2-hydroxyethyl methacrylate) using natural deep eutectic solvents for sustainable cancer drug delivery. SN Appl Sci 1(568):1–13
Behari K, Tripathi M, Taunk K, Kumar R (2000) Studies of graft copolymerization of acrylamide onto guar gum using peroxy diphosphate–metabisulphite redox pair. Polym Int 49:153–157
Bowman LM, Cha CY (2003) Solution properties of poly (N, N-diallyldimethyl ammoniumchloride). J Polym Sci Polym Lett Ed 17:167–173
Liu Q, Li J, Xu W (2011) Preparation and application of starch-acrylamide-diallyldimethylammonium chloride graft copolymer. Mater Sci Forum 675–677:403–406
Mclean D, Agarwal V, Stack K, Horne J, Richerdson D (2011) Synthesis of gurgum- graft(acrylamide-co-diallyldimethylammoniumchloride) and its application in the pulp and paper industry. Bio Resour 6(4):4168–4180
Naidu BVK, Paulson AT (2011) A new method for the preparation of gelatin nanoparticles: encapsulation efficiency and drug release characteristics. J Appl Poly Sci 121:3495–3500
Rai G, Awesh KY, Narendra KJ, Govind PA (2014) Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv 23(1):1–10
Eun SG, Jianshu L, Huining X, Tao LL (2009) Quaternary ammonium -cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules 10:505–516
Natassa P, Maria K, Stergios P, Costas D (2015) Complexation of cationic-neutral block polyelectrolyte with insulin and in vitro release studies. Int J Pharm 491:136–143
Yan-Hsung W, Yin-Chih F, Hui-Chi C, Chau-Zen W, Shao-Ping L, Mei-Ling H, Po-Len L, Chih-Kuang W (2013) Cationic nanoparticles with quaternary ammonium-functionalized PLGA–PEG-based copolymers for potent gene transfection. J Nanopart Res 15:2077
Sonia TA, Chandra PS (2013) N-hydroxy propyl trimethyl ammonium poly dimethyl amino ethyl methacrylate sub-microparticles for oral delivery of insulin-An in vitro evaluation. Colloids Surf B 107:205–212
Longfei L, Lin C, Huan Z, Yongzhen Y, Liu X, Yongkang C (2016) Temperature and magnetism bi-responsive molecularly imprinted polymers: preparation, adsorption mechanism and properties as drug delivery system for sustained release of 5-fluorouracil. Mater Sci Eng C 61:158–168
Lin C, Li L, Huan Z, Weifeng L, Yongzhen Y, Liu X, Xu B (2014) Magnetic thermosensitive core/shell microspheres: synthesis, characterization and performance in hyperthermia and drug delivery. RSC Adv 4:46806–46812
Shalin PT, Ajit PR, Shivayya SA, Sudhir RI, Venkatarao HK, Uttam AM, Tejraj MA (2014) Inter-polymer complex microspheres of chitosan and cellulose acetate phthalate for oral deliveryof 5-fluorouracil. Polym Bull 71:2113–2131
Fang Z, Weibing W, Xiaodan Z, Xianzhi M, Guolin T, Yulin D (2016) Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 23:415–425
Changyou G, Stefano L, Sergio M, Edwin D, Helmuth M (2001) Stability and mechanical properties of polyelectrolyte capsules obtained by stepwise Assembly of poly (styrenesulfonate sodium salt) and poly (diallyl dimethyl ammonium) chloride onto melamine resin particles. Langmuir 17:3491–3495
Ramos J, Forcada J, Hidalgo-Alvarez R (2014) Cationic polymer nanoparticles and nanogels: from synthesis to biotechnological applications. Chem Rev 114(1):367–428
Adeline S, Hang L, Marion T, Paul G, Andreas S, Ijeoma U (2012) Enhanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol Pharm 9:14–28
Zaid AA, Athir MH (2018) Preparation of polyelectrolyte hydrogels and study their controlled release of gabapentin. Prop Int J 1(2):55–63
Acknowledgements
Authors are sincerely thankful to Department of Science and Technology, New Delhi for providing grant (SR/FT/CS-70/2010). We thankful to Dr. Krishna Rao, Dept. of Chemistry, Yogi Vemana University, Kadapa for providing UV–Visible Spectrophotometer, FTIR and tablet dissolution tester facility and also Dr. V. Ramakrishna, Dept. of Biotechnology and Bioinformatics, Yogi Vemana University, Kadapa for helping in hemolysis and cytotoxic studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sana, S.S., Arla, S.K., Badineni, V. et al. Development of poly (acrylamide-co-diallyldimethylammoniumchloride) nanogels and study of their ability as drug delivery devices. SN Appl. Sci. 1, 1716 (2019). https://doi.org/10.1007/s42452-019-1742-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1742-3