Abstract
Electrochemical dissolution behavior of aluminum foil in acid etchants was clearly investigated with different temperatures. Due to the electrochemical activation of temperature, the surface dissolution of aluminum foil in H2SO4 etchant slightly increased with the temperature. Even if NaCl was contained in the H2SO4 solution, the dissolution of aluminum surface and the value of Epit were dependent on the temperature. The rising temperature decreased the resistance of pitting on aluminum foil surface, which enhancing the density and uniformity of the tunnel pits. The surface dissolution and tunnel etching behavior of aluminum foil was discussed by a schematic diagram.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Direct current (DC) tunnel pitting of (100)-oriented aluminum foil in chloride ion-containing solution has been usually used for enlargement of the specific surface area of aluminum foil used in high-voltage electrolytic capacitors [1,2,3,4]. A high density of tunnel pits can be generated on aluminum surface by using the DC etching [5,6,7,8,9]. Because the capacitance of the etched aluminum foil is primarily decided by the specific surface area, the distribution and size of the tunnel pits on etched aluminum surface must be generated uniformly to enhance the specific surface area [10,11,12,13,14,15,16,17,18]. The effects of concentration, components, and temperature on the etched tunnel pits have been well investigated [12, 19,20,21,22,23].
Electrochemical etching at temperature of 70–95 °C is currently carried out in chloride ion-containing solutions, such as NaCl and HCl [24]. The investigation of temperature on etched tunnel growth on aluminum foil surface has been studied significantly. Osawa and Fukuoka [3] investigated the effects of temperature on the tunnel shape growth, and indicated that the time for etched tunnels to initiate decreased as temperature increases. At 70 °C, etched tunnels were first seen at about 250 ms. Alwitt et al. indicated that the steady state tunnel growth occurred at constant rate with activation energy of 15 kcal mol−1, from 97 to 50 °C [19, 25]. Makino et al. also investigated the steady state tunnel growth at temperatures below 60 °C [12, 26]. However, the surface dissolution of aluminum foil in various etchant at different temperature was few investigated. Currently, the surface dissolution is synchronously occurred on aluminum surface and into the etched tunnels.
In this paper, DC etching of aluminum foil was carried out in etchants, and the evaluation of temperature on the electrochemical dissolution of etched aluminum foil was studied clearly.
2 Experimental
In this work, aluminium foil (JOINWORLD,China) used was 99.99 wt% pure and 120 μm thickness. The foil was fully annealed so that the {100} cubicity texture fraction was above 95%. The aluminum foil was firstly handled in a solution of 1.5 M NaOH at 50 °C for 40 s. An electrochemical tank of three electrodes was used for the electrochemical testing of the as-received aluminum foil in 3.5 M H2SO4 solutions with or without NaCl at different temperature. The testing area of the as-received aluminum foil was 1 cm2 by coating epoxy resin and transferred into etchant for etching. The working electrode (WE), counter electrode (CE) and reference electrode (RE) were performed by the aluminum foil, a Pt foil and a saturated calomel electrode (SCE), respectively [7]. A scanning rate of 15 mV s−1 was used to test the potentiodynamic polarization curve. And a current density of 200 mA cm−2 was used to test the initial potential transient. Electrochemical impedance spectroscopy (EIS) was tested in the various frequency.
The surface morphologies were observed by scanning electron microscope (SEM, JSM-6610A). To observe the etched tunnels easily, the etched specimens were electropolished using voltage of 18 V in solutions of 15 vol% HClO4 and 85 vol% ethanol at 0 °C for 25 s. The density, size and uniformity of the tunnels on aluminum surface were analyzed clearly based on the images.
3 Results and discussion
3.1 Etching solution without NaCl
The potentiodynamic polarization of aluminum foil etched in 3.5 M H2SO4 solution at different temperature is shown in Fig. 1. In the positive region of potential, the current density slightly increased with the increasing solution temperature. Figure 2 shows the Nyquist plots of aluminum foil etched in the same condition. With the increasing solution temperature, the capacitance loop in Fig. 2 gradually becomes smaller. In order to evaluate the corrosion resistance of the etched aluminum foil, the impedance curves were studied using the fitting equivalent circuit, which is appeared in the inset of Fig. 2. It composed of the electrolyte resistance (Rs), the resistance of surface corrosion (Rct) as well as a double layer capacitance (Cdl). The evaluated parameters based on the equivalent circuit model are given in Table 1. It can be expected from the parameters that the value of Rct slowly reduced with increasing solution temperature. It indicates that the increasing solution temperature enhance the uniform dissolution of the native oxide film.
3.2 Etching solution with NaCl
The essential polarization of etched aluminum foil in solutions of H2SO4 and NaCl is shown in Fig. 3. Tunnel pits of the aluminum surface were initiated as the potential gradually raised to the critical pitting potential (Epit). Under Epit, the maximum value of the current density (ic) is decided by lengthening the curve to B point. The current density increased rapidly when pitting was initiated.
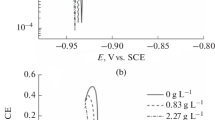
In various solutions, the polarization curves of aluminum foil etched at different temperature is shown in Fig. 4. When the scanning potential is lower than the value of Epit, the polarization curves tested in the solutions of 3.5 M H2SO4 + 0.5 M NaCl at different temperature were almost coincided with those tested in the H2SO4 solution without NaCl. This novel result implies that the surface dissolution was independent on the solution temperature even if etching were carried out in solutions contained NaCl. The added NaCl hardly affected the surface dissolution of etched aluminum foil. Besides, the increasing temperature made the value of Epit gradually decreased, which indicates that the initiation of etched pits become easier. The evaluation of Epit at different temperature was depicted in Fig. 5. The value of Epit is reduced linearly with increased temperature. It indicates that the value of Epit is directly determined by the extent of surface dissolution of aluminum foil.
At 75 °C, the polarization curves of aluminum foil etched at different concentration of NaCl is shown in Fig. 6. Under the values of Epit, the polarization curves tested in the solutions of 3.5 M H2SO4 + n M NaCl (n = 0.25, 0.5 and 0.75) at 75 °C were also coincided with the curve tested in the 3.5 M H2SO4 solution without NaCl at 75 °C. This result implies that the surface dissolution was independent on the NaCl concentration. The added NaCl hardly affected the surface dissolution of etched aluminum foil. However, it is well known that the increased NaCl concentration can reduce the the value of Epit.
The initial potential transient of etched aluminum foil in 3.5 M H2SO4 + 0.5 M NaCl solution at different temperature is shown in Fig. 7. The potential, induced by the ohmic drop and the electric double layer, rapidly increased from the initial potential. Then, due to the appearance of the film, the potential slightly rasied to the maximum value (Em). The duration of potential increase (τa) gradually decreased with increasing solution temperature. The breakdown potential of the native oxide film can be estimated by the maximum potential Em [27, 28]. It can be seen that Em and τa slowly reduced with increased solution temperature, which indicated that the native oxide film dissolved more intensively with temperature. The gradually decreased oxide film can expose more and more aluminum surface to pitting initiation.
3.3 Morphologies of etched aluminum foil
Figure 8 shows the scanning electron micrographs of etched aluminum surface in solutions of 3.5 M H2SO4 + 0.5 M NaCl at different temperature for 95 s. With the increasing solution temperature, the density of the etched tunnels gradually increased and the distribution of the etched tunnels slowly uniform. In order to evaluate the surface morphologies clearly, the statistics of etched tunnels were shown in Fig. 9. A normal distribution of the sizes of etched tunnels can be seen in Fig. 9. The average size and density of etched tunnels is 0.44 μm and 2.08 × 107 cm−2 for 70 °C, and 0.41 μm and 2.61 × 107 cm−2 for 75 °C, and 0.33 μm and 4.3 × 108 cm−2 for 80 °C, respectively. The evaluated data for the etched aluminum foil at different temperature are listed in Table 2, where ρs is the corroded area per unit surface area. The above results imply that with the increasing solution temperature, the distribution and density of the etched tunnels will be improved largely. Besides, the corroded area per unit surface area (ρs) is improved with increased solution temperature, implies that the enhanced specific capacitance.
Size distribution of etched tunnels based on the surface images of Fig. 7: a 70 °C, b 75 °C and c 80 °C
3.4 Interpretation of the etching processes
As shown in Fig. 10, based on the schematic diagram, the effect of solution temperature on the native oxide film and etched tunnels of aluminum foil were analyzed. To obtain high {100} cube texture which is necessary for tunnel etching of high-voltage aluminum foil, aluminum foil should be annealed at 500 °C for a long time. As shown in Fig. 10(a-1), (b-1), the annealed treatment will inevitably result in the formation of native oxide film on the aluminum foil surface. The surface dissolution etching depended mainly on the solution temperature and was hardly affected by the added NaCl, which has been proved by the polarization curves in Fig. 4. Because the increasing solution temperature promote the activity of ions, the dissolution of the native oxide film generated on aluminum surface is accelerated, which implies that a thinner oxide film is obtained, as shown in Fig. 10(b-2). So at high temperature, Cl− can penetrate lightly to aluminum surface and initiate tunnel pitting. At last, the density and uniformity of the etched tunnels are improved significantly, as shown in Fig. 10(b-3).
4 Conclusions
Electrochemical dissolution of etched aluminum foil was carried out in solutions of 3.5 M H2SO4 solution with or without NaCl at different temperature. The effects of solution temperature on the dissolution behavior were studied clearly. The relevant conclusions were described as follows:
-
(1)
The solution temperature decided the surface dissolution of aluminum surface, and the added NaCl would not affect it. A surface of lower resistance could be generated with increasing temperature to improve the pitting initiation.
-
(2)
The size and uniformity of the etched tunnels on etched aluminum foil were improved obviously with increased solution temperature, which was helpful for enhancing the specific surface area.
-
(3)
A schematic diagram was proposed to evaluate the effect of solution temperature on the surface dissolution and dissolution behavior of aluminum foil.
References
Yao L, Liu J, Li S, Yu M (2014) Effects of prior cathodic polarization on crystallographic pit initiation on aluminum. Corros Sci 80:12–18
Xiao R, Yan K, Yan J, Wang J (2008) Electrochemical etching model in aluminum foil for capacitor. Corros Sci 50:1576–1583
Osawa N, Fukuoka K (2000) Pit nucleation behavior of aluminium foil for electrolytic capacitors during early stage of DC etching. Corros Sci 42:585–597
Peng N, He Y, Yang H (2018) Effects of H2SO4 content on electrochemical activation of etched tunnels on aluminum foil. Corrosion 74(1):75–82
Liang L, He Y, Song H, Yang X, Cai X, Xiong C, Li Y (2013) Effect of hydration pretreatment on tunnel etching behaviour of aluminium foil. Corros Sci 70:180–187
Liang L, He Y, Song H, Yang X, Cai X (2014) Effect of placement of aluminium foil on growth of etch tunnels during DC etching. Corros Sci 79:21–28
Ban C, He Y, Shao X (2013) Effects of polymer corrosion inhibitor on widening etch tunnels of aluminum foil for capacitor. Corros Sci 78:7–12
Ashitaka Z, Thompson GE, Skeldon P, Wood GC, Shimizu K (1999) The behavior of copper and lead during heat treatment and surface treatment of aluminium foils. J Electrochem Soc 146:1380–1385
Ashitaka Z, Skeldon P, Thompson GE, Shimizu K, Habazaki H (2002) Enrichment behaviour of gallium in heat and surface treatments of Al–Ga foils. Corros Sci 44:2725–2735
Asoh H, Nakamura K, Ono S (2007) Control of pit initiation sites on aluminum foil using colloidal crystals as mask. Electrochim Acta 53:83–86
Lee J, Kim J, Kim J, Lee J, Chung H, Tak Y (2009) Effects of pretreatment on the aluminium etch pit formation. Corros Sci 51:1501–1505
Makino T, Alwitt RS, Ono S (2007) Growth of etch tunnels in aluminum at temperatures of 28–60 °C. J Electrochem Soc 154:132–137
Fukushima T, Nishio K, Masuda H (2010) Optimization of etching conditions for site-controlled tunnel pits with high aspect ratios in Al foil. J Electrochem Soc 157:137–139
Baumgartner M, Kaesche H (1990) Aluminum pitting in chloride solutions: morphology and pit growth kinetics. Corros Sci 31:231–236
Matsuki K, Tachibana K, Sugawara M, Funakoshi A, Suganuma E (1988) Study on AC etching of aluminum in hydrochloric acid solution by cyclic chronopotentiometry. J Metal Finish Soc Jpn 39:522–536
Makino E, Yajima T, Shibata T, Ikeda M, Tanno Y, Suganuma E (1993) In situ observation of growing pits during tunnel etching of aluminum. Mater Trans JIM 34:796–800
Peng N, He Y, Song H, Yang X, Cai X (2015) Effects of electrodeposited Zn nuclei on tunnel etching behavior of aluminum foil. Corros Sci 91:213–219
Zhu SQ, Ban CL, Tao XQ, Chen WY, Jiang LJ (2015) Modelling specific capacitance of DC etched aluminium foil for aluminium electrolytic capacitor. J Mater Sci Mater Electron 26(9):6750–6756
Alwitt RS, Uchi H (1984) Electrochemical tunnel etching of aluminum. J Electrochem Soc 131:13–17
Muthukrishnan K, Hebert KR (2004) Kinetic model for aluminum dissolution in corrosion pits. J Electrochem Soc 151:45–52
Hebert KR (2001) A mathematical model for the growth of aluminum etch tunnels. J Electrochem Soc 148:236–243
Tak Y, Sinha N, Hebert KR (2000) Metal dissolution kinetics in aluminum etch tunnels. J Electrochem Soc 147:4103–4112
Peng N, Liang LB, He YD, Song HZ, Yang XF, Cai XY (2014) Effect of tunnel structure on the specific capacitance of etched aluminum foil. Int J Miner Metall Mater 21:974–979
Na KH, Pyun SI (2007) Effects of sulphate, nitrate and phosphate on pit initiation of pure aluminium in HCl-based solution. Corros Sci 49:2663–2675
Alwitt RS, Hebert KR, Makino T (2002) Etch tunnels in aluminum, vol 20, pp 223–226
Ui K, Yamasaki T, Koura N, Idemoto Y, Makino T, Furukawa M, Uchi H (2006) Growth model of reversed taper during early stage of DC etching on aluminum oriented to (100). Electrochemistry 74:737–743
Ryu J-H, Seo JH, Jeong J-H, Kim S-K, Lee DN (2004) The effect of aluminum ions on the DC etching of aluminum foil. J Appl Electrochem 34:879–884
Flis J, Kowalczyk L (1995) Effect of sulphate anions on tunnel etching of aluminium. J Appl Electrochem 25:501–507
Acknowledgements
The authors acknowledged the financial support by the National Natural Science Foundation of China (No. 51665010) and the Guangxi Key Laboratory of Electrochemical and Magnetochemical Functional Materials (No. EMFM20181106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have not any financial/commercial conflicts of interest.
Rights and permissions
About this article
Cite this article
Peng, N., Wen, Y. & He, Y. Effects of temperature on electrochemical dissolution behavior of aluminum foil. SN Appl. Sci. 1, 88 (2019). https://doi.org/10.1007/s42452-018-0090-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0090-z