Abstract
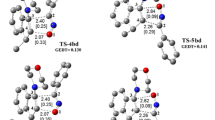
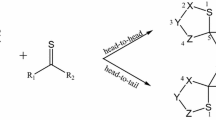
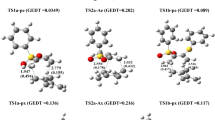
The 1,3-dipolar cycloaddition reactions also known as the Huisgen cycloaddition are one of the most widely used and versatile preparative methods in hetrocyclic chemistry. In this study, the reactivity and strain energy effect of the simple cycloalkynes with substituted Nitrile sulfides R–CNS (R = H, CH3, Ph and Ph(CH3)3) will be discussed in light of computational studies using DFT methods (B3LYP/6-31G*). The investigation of the structured properties, theoretical thermodynamic and kinetic data of the reactions in 298 K will be presented. The results show increase in the ∆G* by increasing the ring size and decreasing the strain energy of cycloalkynes. Also, the rate constants and the free energy changes in reactions increase as the size of the ring decreases. The relationships of the deviation of the internal bond angle (DIBA, in degrees), π-strain (S π ) (in kcal mol−1), the bond angle of Csp3–C ≡ C (θº) and ΔG #(kcal mol−1) for the series of cycloalkynes I-1 to I-4 have investigated.









Similar content being viewed by others
References
Bak B, Christianseen JJ, Nieleen OJ, Svanholt H (1977) Acta Chem Scand A31:666
Carey FA, Sundberg RJ (2007) Advanced organic chemistry, 5th edn. Springer Science, New York
Dirks AJ, van Berkel SS, Hatzakis NS, Opsteen JA, van Delft FL, Cornelissen JJLM, Rowan AE, van Hest JCM, Rutjes FPJT, Nolte RJM (2005) Chem Commun 33:4172–4174 (and the literature cited there in)
Domingo LR, Pe´rez P, Contreras R (2006) Eur J Org Chem 2:498–506
Ess DH, Houk KN (2008) J Am Chem Soc 130:10187–10198
Ess DH, Jones GO, Houk KN (2006) Adv Synth Catal 348:2337–2361
Ess DH, Hayden AE, Kla¨rner FG, Houk KN (2008) J Org Chem 73:7586–7759
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, PomelliC Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98 Revision A.7. Gaussian Inc., Pittsburgh
Huisgen R (1961) Cenetary lecture-1,3-dipolar cycloadditions. In: Proceedings of the chemical society of London, p 357
Huisgen R (1963) Angew Chem Int Ed 2(10):565–598
Huisgen R, Hauck H, Gashey R, Seidl H (1968a) Chem Ber 101:2568–2584
Huisgen R, Gashey R, Hauck H, Seidl H (1968b) Chem Ber 101:2548–2558
Huisgen R, Gashey R, Hauck H (1968c) Seidl H Chem Ber 101:2559–2567
Johnson RP, Daoust KJ (1995) J Am Chem Soc 117:362
Lan Y, Zou L, Cao Y, Houk KN (2011) J Phys Chem A 115:13906–13920
McQuarrie DA, Simon JD (1999) Physical chemistry. University Science Books, Sausalito
Patton GC (2004) Development and applications of click chemistry. http://www.chemistry.illinois.edu/research/organic/seminar_extracts/2004_2005/08_patton_abstract.pdf. Accessed 8 Nov 2004. (and the literature cited there in)
Sakai S, Nguyen MT (2004) J Phys Chem A 108(42):9169–9179
Taherpour AA, Faraji M (2008) Molbank M 577
Taherpour AA, Kheradmand K (2009) J Heterocyclic Chem 46:131–133
Taherpour AA, Kvaskoff D, Bernhardt PV, Wentrup C (2010) J Phys Org Chem 23:382–389
Taherpour AA, Shfeei H, Rajaeian E (2011) Orient J Chem 27(3):885–893
Taherpour AA, Rajaeian E, Shafiei H, Malekdar M (2013) Struct Chem 24(2):523–534
Van Steenis DJVC, David ORP, van Strijdonck GPF, van Maarseveen JH (2005) Reek JNH Chem Commun 34:4333–4335
Vollhardt KPC (1987) Organic chemistry. W. H. Freeman & Company, New York
Xu L, Doubleday CE (2010) Houk KN J Am Chem Soc 132:3029–3037
Acknowledgments
The corresponding author gratefully acknowledges from Theoretical and Computational Research Center of Chemistry Faculty of Razi University-Kermanshah-Iran, The authors gratefully acknowledge the Medical Biology Research Center and Kermanshah University of Medical Sciences, Kermanshah.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajaeian, E., Mirzaei, E. & Taherpour, A.A. Theoretical Calculation of Thermodynamic and Kinetic Quantities for 1,3 Dipolar Cycloaddition Reactions Between Nitrile Sulfides R–CNS (R = H, CH3, Ph and Ph(CH3)3) with 7–10 Membered Simple Cycloalkynes. Iran J Sci Technol Trans Sci 41, 1139–1148 (2017). https://doi.org/10.1007/s40995-016-0053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0053-4