Abstract
Introduction
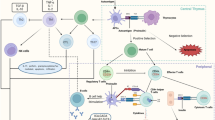
Although many approaches have been tested to overcome the insulin dependence caused by the pancreatic β-cells destruction observed in individuals affected by type 1 diabetes (T1D), medical research has largely failed to halt the onset or to reverse T1D.
Methods
In this work, the state of the art of immunotherapy will be examined, and the most important achievement in the field will be critically discussed. Particularly, we will focus on the clinical aspect, thus avoiding the tedious preclinical work done in NOD mice, which has been so poorly translated to the bedside.
Conclusions
Stem cell therapies achieved thus this far the most promising results, while immune ablation and standard immunosuppressants did not maintain the premises of preclinical results. The next step will be to generate a feasible and safe clinical approach in order to cure the thousands of patients affected by T1D.
Similar content being viewed by others
References
American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32(Suppl. 1):62–67
Jin P, Xiang B, Huang G, Zhou Z (2015) The association of cytotoxic T-lymphocyte antigen-4 + 49/AG and CT60 polymorphisms with type 1 diabetes and latent autoimmune diabetes in Chinese adults. J Endocrinol Invest 38(2):149–154
Bellastella G, Maiorino MI, Petrizzo M, De Bellis A, Capuano A, Esposito K, Giugliano D (2015) Vitamin D and autoimmunity: what happens in autoiimune polyendocrine syndromes? J Endocrinol Invest 38(6):629–633
Talaat IM, Nasr A, Alsulaimani AA, Alghamdi H, Alswat KA, Almalki DM, Abushouk A, Saleh AM, Alla G (2016) Association between type 1, type 2 cytokines, diabetic autoantibodies and 25-hydroxivitamin D in children with type 1 diabetes. J Endocrinol Invest 39(12):1425–1434
Fadini GP, Ciciliot S, Albiero M (2017) Review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells 35(1):106–116
Ben Nasr M, D’Addio F, Usuelli V, Tezza S, Abdi R, Fiorina P (2015) The rise, fall, and resurgence of immunotherapy in type 1 diabetes. Pharmacol Res 98:31–38
Kemink SAG, Hermus ARMM, Swinkles LMJW, Lutterman JA, Smals AGH (2000) Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest 23(5):295–303
Astorri E, Fiorina P, Gavaruzzi G, Astorri A, Magnati G (1997) Left ventricular function in insulin-dependent and in non-insulin-dependent diabetic patients: radionuclide assessment. Cardiology 88(2):152–155
Zhukouskaya VV, Eller-Vainicher C, Shepelkevich AP, Dydyshko Y, Cairoli E, Chiodini I (2015) Bone health in type 1 diabetes: focus on evaluation and treatment in clinical practice. J Endocrinol Invest 38(9):941–950
Cizza G, Brown RJ, Rother KI (2012) Rising incidence and challenges of childhood diabetes. A mini review. J Endocrinol Invest 35(5):541–546
Maltoni G, Zucchini S, Scipione M, Rollo A, Balsamo C, Bertolini C, Baronio F, Rondelli R, Pession A (2013) Severe hypoglycemic episodes: a persistent threat for children with type 1 diabetes mellitus and their families. J Endocrinol Invest 36(8):617–621
Rewers M, Gottlieb P (2009) Immunotherapy for the prevention and treatment of type 1 diabetes. Diabetes Care 32(10):1769–1782
Perseghin G, Fiorina P, De Cobelli F, Scifo P, Esposito A, Canu T, Danna M, Gremizzi C, Secchi A, Luzi L, Del Maschio A (2005) Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic-uremic patients: a phosphorous-31 magnetic resonance spectroscopy study. J Am Coll Cardiol 46(6):1085–1092
Miladpour B, Rasti M, Owji AA, Mostafavipour Z, Khoshdel Z, Noorafshan A, Zal F (2016) Quercetin potentiates transdifferentiation of bone marrow mesenchymal stem cells into the beta cells in vitro. J Endocrinol Invest. doi:10.1007/s40618-016-0592-8
Kung P, Goldstein G, Reinherz EL, Schlossman SF (1979) Monoclonal antibodies defining distinctive human T cell surface antigens. Science 206(4416):347–349
Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, Russell PS (1981) Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation 32(6):535–539
Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone J (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N engl J Med 346:1692–1698
Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L (2005) Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N engl J Med 352(25):2598–2608
Herold K, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA, Group Immune Tolerance Network ITN007AI Study (2009) Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 132 (2): 166–173
Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomized, placebo-controlled trial. The Lancet 378(9790):487–497
Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA, AbATE Study Team (2013) Teplizumab (Anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62(11):3766–3774
Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM (2014) Efficacy and safety of low-dose Otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabetic Med 31 (4):399–402.
Vergani A, D’Addio F, Jurewicz M, Petrelli A, Watanabe T, Liu K, Law K, Schuetz C, Carvello M, Orsenigo E, Deng S, Rodig SJ, Ansari JM, Staudacher C, Abdi R, Williams J, Markmann J, Atkinson M, Sayegh MH, Fiorina P (2010) A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes 59(9):2253–2264
Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Harris KM, Kanaparthi S, Phippard D, Ding L, Bluestone JA, Ehlers MR (2016) Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 years results of a randomized trial. Diabetologia 59(6):1153–1161
Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA (2015) Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 125(1):448–455
Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, Wu E, Tian Z, Abdi R, Guleri I, Rodig S, Dunussi-Joannopoulos K, Bluestone J, Sayegh MH (2008) Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 57:3013–3024
Hu C, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomichik MJ, Wen L (2007) Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 117:3857–3867
Pescovitz MD, Grrenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wehrret D, Wilson DM, Lachen JM, Skyler JS (2009) Rituximab, B-Lymphocyte depletion, and preservation of beta-cell function. N engl J Med 361:2143–2152
Diabetes Prevention Trial—Type 1 Diabetes Study Group (2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N engl J Med 346(22):1685–1691
Diabetes Prevention Trial - Type 1 Diabetes Study Group (2005) Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care 28(5):1068–1076
Wherrett DK, Bundy B, Becker DJ, Di Meglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Palmer JP, Raskin P, Rodriguez H, Schatz D, Wilson DM, Krischer JP, Skyler JS, Type 1 Diabetes Trial Net GAD Study Group (2011). Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomized double-blind trial. Lancet 378(9788):319–27.
Crinò A, Schiaffini R, Manfrini S, Mesturino C, Visalli N, Beretta Anguissola G, Suraci C, Pitocco D, Spera S, Corbi S, Matteoli MC, Patera IP, manca Bitti ML, Bizzarri C, Pozzilion P (2004). A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX). Eur J Endocrinol 150:719–724
Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, Schatz DA, Moran AM, Lachin JM, Skyler JS (2010) Failure to preserve β-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care 33(4):826–832
Sobel DO, Henzke A, Abbassi V (2010) Cyclosporin and methotrexate therapy induces remission in type 1 diabetes mellitus. Acta Diabetol 47(3):243–250
Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS, Type 1 Diabetes TrialNet Canakinumab Study Group, Pickersgill L, de Koning E, Ziegler AG, Böehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castaño L, Wägner A, Lervang HH, Perrild H, Mandrup-Poulsen T, AIDA Study Group (2013). Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381 (9881): 1905–1915.
Kalliolias GD, Liossis SN (2008) The future of IL-1 receptor antagonist anakinra: from rheumatoid arthritis to adult-onset Still’s disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs 17(3):349–359
Van Asseldonk EJ, Van Poppell PC, Ballak DB, Stienstra R, Netea MG, Tack CJ (2015). One week treatment with the IL-1 receptor antagonist Anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin Immunol 160 (2):155–162.
Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, Bassi R, D’Addio F, SchattonT, Abdi R, AtkinsonM, Sayegh MH, Wen L, Wasserfall CH, O’Connor KC, Fiorina P (2015) Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes 64(1):158–171
Fiorina P, Jurewicz M, Tanaka K, Behazin N, Augello A, Vergani A, Von Adrian U, Smith NR, Sayegh MH, Abdi R (2007) Characterization of donor dendritic cells and enhancement of dendritic cell efflux with cc-chemokine ligand 21. A novel strategy to prolong islet allograft survival. Diabetes 56(4):912–920
Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF (2013) Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 14(4):307–308
Giannoukakis N, Trucco M (2012) Dendritic cell therapy for type 1 diabetes suppression. Immunotherapy 4 (10):1063–1074.
Fiorina P, Jurewicz M, Tanaka K, Behazin N, Augello A, Vergani A, Adrian UV, Smith NR, Sayegh MH, Abdi R (2007) Characterization of donor dendritic cells and enhancement of dendritic cell efflux with cc-Chemokine ligand 21. Diabetes 56(4):912–920
Giannouakis N, Phillips B, Finegold D, Harnah J, Trucco M (2011) Phase I (Safety) study of 12 autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 34:2026–2032
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7(315):1–14
Chhabra P, Brayman KL (2013) Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med 2(5):328–336.
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R (2009) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183(2):993–1004
D’Addio F, Trevisani A, Ben Nasr M, Bassi R, El Essawy B, Abdi R, Secchi A, Fiorina P (2014) Harnessing the immunological properties of stem cells as a therapeutic option for diabetic nephropathy. Acta Diabetol 51(6):897–904
Jurewicz M, Sunmi Y, Augello A, Godwin JG, Moore RF, Azzi J, Fiorina P, Atkinson M, Sayegh MH, Abdi R (2010) Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 59(12):3139–3147
Fiorina P, Voltarelli, Zavazava N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev 32(6):725–754
Carlsson PO, Schwarz E, Korsgren O, Le Blanc K (2015) Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64(2):587–592
Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, Pileggi A, Ricordi C, Tan J (2016) Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 39(1):149–157
Voltarelli JC, Couri CE, Starcieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simoes BP, Foss MC, Squiers E, Burt RK (2007) Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14):1568–1576
D’Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P (2014) Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes 63:3041–3046
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent in not required.
Rights and permissions
About this article
Cite this article
Frumento, D., Ben Nasr, M., El Essawy, B. et al. Immunotherapy for type 1 diabetes. J Endocrinol Invest 40, 803–814 (2017). https://doi.org/10.1007/s40618-017-0641-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0641-y