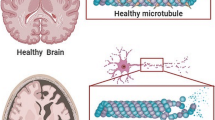
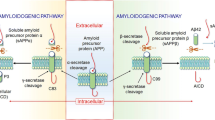
Abstract
Purpose of Review
A number of studies over the past two decades have suggested that type 2 diabetes mellitus (T2DM) patients are at an increased risk of Alzheimer’s disease (AD). Several common molecular pathways to cellular and metabolic dysfunction have been implicated in the etiology of both diseases. Here, we review the emerging evidence from observational studies that investigate the relationship between T2DM and AD, and of shared environmental risk factors, specifically air pollution and pesticides, associated with both chronic disorders.
Recent Findings
Particulate matter and traffic-related air pollution have been widely associated with T2DM, and multiple studies have associated exposures with AD or cognitive function. Organochlorine (OC) and organophosphate (OP) pesticides have been associated with T2DM in multiple independent populations. Two populations have observed increased risks for OC and OP exposures and AD. Other studies, limited in exposure assessment, have reported increased risk of AD with any pesticide exposure assessments.
Summary
This may suggest shared pathogenic pathways between environmental risk factors, T2DM, and AD. Research focusing on exposures related to both T2DM and AD could provide new disease insights on shared mechanisms and help shape innovative preventative measures and policy decisions.

Similar content being viewed by others
Abbreviations
- DDT:
-
Dichlorodiphenyltrichloroethane
- DDE:
-
Dichlorodiphenyldichloroethylene
- HCB:
-
Hexachlorobenzene
- ppb:
-
Parts per billion
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
United Nations. Report of the Second World Assembly on Ageing: Madrid, 8-12 April 2002. United Nations Publications; 2002. http://www.un.org/en/ga/search/view_doc.asp?symbol=A/CONF.197/9.
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. https://doi.org/10.1016/j.jalz.2012.11.007.
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. https://doi.org/10.1016/j.diabres.2011.10.029.
Han W, Li C. Linking type 2 diabetes and Alzheimer’s disease. Proc Natl Acad Sci. 2010;107(15):6557–8. https://doi.org/10.1073/pnas.1002555107.
Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM. Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci. 2017;40(6):347–57. https://doi.org/10.1016/j.tins.2017.04.002.
Morrison CD. Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta Mol Basis Dis. 2009;1792(5):401–8. https://doi.org/10.1016/j.bbadis.2008.12.004.
Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177(1–2):125–34. https://doi.org/10.1016/S0303-7207(01)00455-5.
Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–19. https://doi.org/10.1016/j.neuron.2008.04.014.
Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7(6):643–7. https://doi.org/10.1016/j.coph.2007.10.006.
Eckman EA, Eckman CB. Abeta-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans. 2005;33(Pt 5):1101–5. https://doi.org/10.1042/BST20051101.
Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162–7. https://doi.org/10.1073/pnas.0230450100.
FEWLASS DC. Obesity-related leptin regulates Alzheimer’s Aβ. FASEB J. 2004;18(15):1870–8. https://doi.org/10.1096/fj.04-2572com.
Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423(lane 2):435–9. https://doi.org/10.1038/nature01640.
Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and a deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci. 2010;107(15):7036–41. https://doi.org/10.1073/pnas.1000645107.
• de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes—evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–13. https://doi.org/10.1177/193229680800200619. This article reviews the literature pointing toward insulin deficiency and insulin resistance as mediators of AD-type neurodegeneration.
Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, et al. Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes. 2015;64(3):1011–24. https://doi.org/10.2337/db13-1181.
Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, et al. Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown Norway rats. Toxicol Appl Pharmacol. 2013;273(3):551–60. https://doi.org/10.1016/j.taap.2013.09.029.
Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53(9):1937–42. https://doi.org/10.1212/WNL.53.9.1937.
MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14(2):77–83. https://doi.org/10.1159/000064928.
Hassing LB, Johansson B, Nilsson SE, et al. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest old. Int Psychogeriatrics. 2002;14(3):239–48. https://doi.org/10.1017/S104161020200844X.
Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. Diabetes. 2002;51(April):1256–62. https://doi.org/10.2337/diabetes.51.4.1256.
Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61(5):661–6. https://doi.org/10.1001/archneur.61.5.661.
Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–6. https://doi.org/10.1212/01.WNL.0000140291.86406.D1.
Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–51. https://doi.org/10.1212/01.wnl.0000172914.08967.dc.
Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63(11):1551–5. https://doi.org/10.1001/archneur.63.11.1551.
Raffaitin C, Gin H, Empana J-P, et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City study. Diabetes Care. 2009;32(1):169–74. https://doi.org/10.2337/dc08-0272.
Al-Emam A, Elhaddad AA, Ramadan E. The risk of clinically diagnosed alzheimer disease in patients with non insulin dependent diabetes mellitus. Egypt J Neurol Psychiatry Neurosurg. 2010;47(3):419–24.
Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75(13):1195–202. https://doi.org/10.1212/WNL.0b013e3181f4d7f8.
Kimm H, Lee PH, Shin YJ, et al. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr 2011;52(3). https://doi.org/10.1016/j.archger.2010.09.004.
Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama Study. Neurology. 2011;77(12):1126–34. https://doi.org/10.1212/WNL.0b013e31822f0435.
Wang K-C, Woung L-C, Tsai M-T, Liu C-C, Su Y-H, Li C-Y. Risk of Alzheimer’s disease in relation to diabetes: a population-based cohort study. Neuroepidemiology. 2012;38(4):237–44. https://doi.org/10.1159/000337428.
Huang C-C, Chung C-M, Leu H-B, Lin LY, Chiu CC, Hsu CY, et al. Diabetes mellitus and the risk of Alzheimer’s disease: a Nationwide population-based study. PLoS One. 2014;9(1):e87095. https://doi.org/10.1371/journal.pone.0087095.
Katon W, Pedersen HS, Ribe AR, Fenger-Grøn M, Davydow D, Waldorff FB, et al. Effect of depression and diabetes mellitus on the risk for dementia: a National Population-Based Cohort Study. JAMA Psychiatry. 2015;98195(6):1–8. https://doi.org/10.1001/jamapsychiatry.2015.0082.
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. https://doi.org/10.2147/CLEP.S91125.
Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35(1):152–60. https://doi.org/10.1093/epirev/mxs012.
Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease. Circulation. 2010;121(21):2331–78. https://doi.org/10.1161/CIR.0b013e3181dbece1.
Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171(5):R173–82. https://doi.org/10.1530/EJE-14-0365.
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14(9):813–22. https://doi.org/10.1016/S1470-2045(13)70279-1.
Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–84. https://doi.org/10.1016/j.neuro.2012.08.014.
Evangelou E, Ntritsos G, Chondrogiorgi M, et al. Exposure to pesticides and diabetes: a systematic review and meta-analysis. Environ Int. 2016;91:60–8. https://doi.org/10.1016/j.envint.2016.02.013.
• Zaganas I, Kapetanaki S, Mastorodemos V, et al. Linking pesticide exposure and dementia: what is the evidence? Toxicology. 2013;307:3–11. https://doi.org/10.1016/j.tox.2013.02.002. This article reviews the epidemiological links between dementia and pesticide exposure and discusses the possible pathophysiological mechanisms and clinical implications of this association.
Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50(1):32–8. https://doi.org/10.1097/JOM.0b013e31815dba70.
Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes in African American women living in Los Angeles. Circulation. 2012. https://doi.org/10.1161/CIRCULATIONAHA.111.052753.
Andersen ZJ, Raaschou-Nielsen O, Ketzel M, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35(1):92–8. https://doi.org/10.2337/dc11-1155.
Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118(9):1273–9. https://doi.org/10.1289/ehp.0901689.
G W, K F, F H, et al. Industry and traffic related air pollution and diabetes type two incidence: results from a German cohort study. Eur J Epidemiol. 2012;27(1 SUPPL. 1):S12. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed14&NEWS=N&AN=71302821
Eze IC, Schaffner E, Fischer E, et al. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int. 2014;70:95–105. https://doi.org/10.1016/j.envint.2014.05.014.
Park SK, Adar SD, O’Neill MS, et al. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am J Epidemiol. 2015;181(5):327–36. https://doi.org/10.1093/aje/kwu280.
Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36(10):3313–20. https://doi.org/10.2337/dc12-2189.
Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. 2013;121(7):804–10. https://doi.org/10.1289/ehp.1205958.
Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119(3):384–9. https://doi.org/10.1289/ehp.1002344.
Weinmayr G, Hennig F, Fuks K, et al. Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: effects of total and traffic-specific air pollution. Environ Health. 2015;14(1):53. https://doi.org/10.1186/s12940-015-0031-x.
Jung C-R, Lin Y-T, Hwang B-F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis. 2015;44(2):573–84. https://doi.org/10.3233/JAD-140855.
Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2015;124(1):23–9. https://doi.org/10.1289/ehp.1408973.
Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219–27. https://doi.org/10.1001/archinternmed.2011.683.
Loop MS, Kent ST, Al-Hamdan MZ, Crosson WL, Estes SM, Estes MG, et al. Fine particulate matter and incident cognitive impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. PLoS One. 2013;8(9):e75001. https://doi.org/10.1371/journal.pone.0075001.
Tonne C, Elbaz A, Beevers S, Singh-Manoux A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology. 2014;25(5):674–81. https://doi.org/10.1097/EDE.0000000000000144.
Schikowski T, Vossoughi M, Vierkötter A, et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res. 2015;142:10–6. https://doi.org/10.1016/j.envres.2015.06.009.
Wu Y-C, Lin Y-C, Yu H-L, Chen JH, Chen TF, Sun Y, et al. Association between air pollutants and dementia risk in the elderly. Alzheimer’s Dement Diagnosis Assess Dis Monit. 2015;1(2):220–8. https://doi.org/10.1016/j.dadm.2014.11.015.
Oudin A, Forsberg B, Adolfsson AN, et al. Traffic-related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect. 2016;124(3):306–12. https://doi.org/10.1289/ehp.1408322.
Chang K-H, Chang M-Y, Muo C-H, Wu T-N, Chen C-Y, Kao C-H. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One. 2014;9(8):e103078. https://doi.org/10.1371/journal.pone.0103078.
Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Envi Health Perspect. 2015;123(5):381–9. https://doi.org/10.1289/ehp.1307823.
Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–2. https://doi.org/10.1016/S1474-4422(16)00067-3.
Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Avron S, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119(5):682–7. https://doi.org/10.1289/ehp.1002767.
Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–58. https://doi.org/10.1056/NEJMoa054409.
Bro-Rasmussen F. Contamination by persistent chemicals in food chain and human health. Sci Total Environ. 1996;188 https://doi.org/10.1016/0048-9697(96)05276-X.
Committee to review the health effects in Vietnam Veterans of exposure to herbicides (Tenth Biennial Update) C, on the Health of select populations B, of Medicine I, of Sciences Engineering, Medicine. Veterans and Agent Orange: Update 2014.; 2016. https://doi.org/10.17226/21845.
Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, DeVito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121(7):774–83. https://doi.org/10.1289/ehp.1205502.
Morgan DP, Lin LI, Saikaly HH. Morbidity and mortality in workers occupationally exposed to pesticides. Arch Environ Contam Toxicol. 1980;9(3):349–82. http://www.ncbi.nlm.nih.gov/pubmed/7396557. https://doi.org/10.1007/BF01057414.
Lee D-H, Lee I-K, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care. 2006;29(7):1638–44. https://doi.org/10.2337/dc06-0543.
Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993-2003. Am J Epidemiol. 2008;167(10):1235–46. https://doi.org/10.1093/aje/kwn028.
Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of great lakes sport fish consumers. Environ Health Perspect. 2009;117(7):1076–82. https://doi.org/10.1289/ehp.0800281.
Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case–control study. Environ Health Perspect. 2010;118(9):1235–42. https://doi.org/10.1289/ehp.0901480.
Lee DH, Lind PM, Jacobs DR, Salihovic S, Van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34(8):1778–84. https://doi.org/10.2337/dc10-2116.
Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses’ health study and meta-analysis. Environ Health Perspect. 2013;121(2):153–61. https://doi.org/10.1289/ehp.1205248.
Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med. 2014;71(9):629–35. https://doi.org/10.1136/oemed-2013-101659.
McDowell I, Hill G, Lindsay J, et al. The Canadian study of health and aging: risk-factors for Alzheimer’s disease in Canada. Neurology. 1994;44(11):2073–80.
Tyas SL, Manfreda J, Strain A, Montgomery PR. Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30(3):590–7. https://doi.org/10.1093/ije/30.3.590.
Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157(5):409–14. https://doi.org/10.1093/aje/kwf216.
Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JCS, et al. Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology. 2010;74(19):1524–30. https://doi.org/10.1212/WNL.0b013e3181dd4423.
Parrón T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol. 2011;256(3):379–85. https://doi.org/10.1016/j.taap.2011.05.006.
Richardson JR, Roy A, Shalat SL, et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014;71(3):284. https://doi.org/10.1001/jamaneurol.2013.6030.
Koeman T, Schouten LJ, van den Brandt PA, Slottje P, Huss A, Peters S, et al. Occupational exposures and risk of dementia-related mortality in the prospective Netherlands Cohort study. Am J Ind Med. 2015;58(6):625–35. https://doi.org/10.1002/ajim.22462.
Lin J-N, Lin C-L, Lin M-C, Lai CH, Lin HH, Yang CH, et al. Increased risk of dementia in patients with acute organophosphate and carbamate poisoning: a Nationwide population-based cohort study. Medicine (Baltimore). 2015;94(29):e1187. https://doi.org/10.1097/MD.0000000000001187.
Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Environmental pesticide exposure as a risk factor for Alzheimer’s disease: a case-control study. Environ Res. 2001;86(1):37–45. https://doi.org/10.1006/enrs.2001.4254.
Jerrett M, Arain A, Kanaroglou P, et al. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol. 2005;15(2):185–204. https://doi.org/10.1038/sj.jea.7500388.
de Hoogh K, Korek M, Vienneau D, et al. Comparing land use regression and dispersion modelling to assess residential exposure to ambient air pollution for epidemiological studies. Environ Int. 2014;73:382–92. https://doi.org/10.1016/j.envint.2014.08.011.
Teschke K, Olshan AF, Daniels JL, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med. 2002;59(9):575–93; discussion 594. https://doi.org/10.1136/oem.59.9.575.
McGuire V, Nelson LM, Koepsell TD, Checkoway H, Longstreth WT. Assessment of occupational exposures in community-based case-control studies. Annu Rev Public Health. 1998;19(19):35–53. https://doi.org/10.1146/annurev.publhealth.19.1.35.
• Brouwer M, Kromhout H, Vermeulen R, et al. Assessment of residential environmental exposure to pesticides from agricultural fields in the Netherlands. J Expo Sci Environ Epidemiol. 2017. This article describes a spatio-temporal model to estimate lifetime exposure to pesticides in the Netherlands based on residential histories.
Milner AM, Boyd IL. Toward pesticidovigilance. Science (80-). 2017;357(6357):1232–4. https://doi.org/10.1126/science.aan2683.
Funding
This work was supported by the National Institute of Environmental Health Sciences (F32-ES028087 (KP), 2R01-ES010544 (BR), R01-ES023451 (BR, MJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Susceptibility Factors in Environmental Health
Rights and permissions
About this article
Cite this article
Paul, K.C., Jerrett, M. & Ritz, B. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Overlapping Biologic Mechanisms and Environmental Risk Factors. Curr Envir Health Rpt 5, 44–58 (2018). https://doi.org/10.1007/s40572-018-0176-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-018-0176-1