Abstract
Background
A chronic low-grade inflammatory profile (CLIP) is associated with sarcopenia in older adults. Protein and Vitamin (Vit)D have immune-modulatory potential, but evidence for effects of nutritional supplementation on CLIP is limited.
Aim
To investigate whether 13 weeks of nutritional supplementation of VitD and leucine-enriched whey protein affected CLIP in subjects enrolled in the PROVIDE-study, as a secondary analysis.
Methods
Sarcopenic adults (low skeletal muscle mass) aged ≥ 65 years with mobility limitations (Short Physical Performance Battery 4–9) and a body mass index of 20–30 kg/m2 were randomly allocated to two daily servings of active (n = 137, including 20 g of whey protein, 3 g of leucine and 800 IU VitD) or isocaloric control product (n = 151) for a double-blind period of 13 weeks. At baseline and after 13 weeks, circulating interleukin (IL)-8, IL-1 receptor antagonist (RA), soluble tumor-necrosis-factor receptor (sTNFR)1, IL-6, high-sensitivity C-reactive protein, pre-albumin and 25-hydroxyvitamin(OH)D were measured. Data-analysis included repeated measures analysis of covariance (corrected for dietary VitD intake) and linear regression.
Results
IL-6 and IL-1Ra serum levels showed overall increases after 13 weeks (p = 0.006 and p < 0.001, respectively). For IL-6 a significant time × treatment interaction (p = 0.046) was observed, with no significant change over time in the active group (p = 0.155) compared to control (significant increase p = 0.012). IL-8 showed an overall significant decrease (p = 0.03). The change in pre-albumin was a significant predictor for changes in IL-6 after 13 weeks.
Conclusions
We conclude that 13 weeks of nutritional supplementation with VitD and leucine-enriched whey protein may attenuate the progression of CLIP in older sarcopenic persons with mobility limitations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is accompanied with a chronic low-grade inflammatory profile (CLIP) reflected by subtle increases in circulating cytokines [1, 2]. CLIP has been associated with frailty in older adults [3, 4], increasing vulnerability to poor health outcomes such as disability, hospital admission and mortality [5]. Sarcopenia, a contributor to physical frailty, is a muscle failure disease that is caused by adverse muscle changes that accumulate over life [6]. A blunted response of muscle protein synthesis to nutrient intake is one of the greatest limitations to muscle preservation, which may be induced by inflammation among other factors [7].
Leucine, a branched-chain amino acid, can induce both anti-inflammatory and pro-inflammatory effects, probably depending on its circulating concentration [8,9,10,11,12]. Previous studies showed that the recommended amount of protein intake for older adults should be 1.0–1.5 g of proteins per kilogram body weight per day [13]. However, a majority of older adults does not reach these amounts of dietary protein intake. Circulating vitamin (Vit)D is inversely related to IL-6 and CRP, and has an anti-inflammatory effect by contributing to the regulation of immune cells [14, 15]. VitD deficiency is often present in older adults due to decreased UV-light exposure and reduced VitD synthesis and metabolism [16]. Therefore, VitD supplementation is often indicated [17].
Studies investigating the anti-inflammatory effect of nutritional supplementation show contradicting results [18,19,20]. In the PROVIDE study, 13 weeks of VitD and leucine-enriched whey protein supplementation improved muscle mass and lower extremity function among sarcopenic older adults [21]. In addition, this study showed that sarcopenic participants with higher baseline circulating VitD concentrations and higher dietary protein intake obtained greater gains in muscle mass after 13 weeks intervention [22]. In the present sub-study, we investigated whether 13 weeks of nutritional supplementation affected circulating inflammatory markers in older sarcopenic adults enrolled in the PROVIDE study.
Materials and methods
Participants
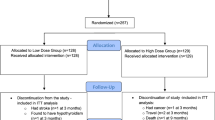
Detailed information on the PROVIDE study protocol was published previously [21] and can be found on https://www.trialregister.nl/trialreg with identifier: NTR2329. Subjects aged ≥ 65 years, with mild to moderate limitations in physical functioning (Short Physical Performance Battery (SPPB) score 4–9), with class I or II sarcopenia (skeletal muscle mass/BW × 100) < 37% in men and < 28% in women using bio-impedance analyses) [23], a body mass index (BMI) of 20–30 kg/m2 and providing written informed consent were eligible for participation [21]. Eligible candidates were evaluated for in -and exclusion criteria during a screening visit as described elsewhere [21]. Briefly, 1240 participants from 6 European countries (Belgium, Germany, Ireland, Italy, Sweden and the United Kingdom) were assessed for eligibility. 380 were randomly allocated by Danone Nutricia Research to either the active (n = 184) or the control group (n = 196) via permuted block randomization (block size 4) stratified for SPPB categories 4–6 and 7–9 and study center. The randomization sequence was computer-generated by a blinded statistician not involved in data collection or analysis. All investigators, study staff, and participants were blinded to group allocations (see Ref. [21] for details). 297 completed the 13 weeks intervention. In total, 78 from the 380 randomized subjects terminated the RCT study early (38 in control and 40 in active group). From the 78 early terminators, 45 subjects did not continue with the study because of an adverse event (AE), 2 subjects did not continue because of serious AE (assessed as not related to the study product), 15 subjects withdrew their informed consent, 2 subjects were lost to follow-up, in 1 subject a protocol deviation occurred (the subject took calcium supplements) leading to study discontinuation and 13 subjects had another reason for discontinuation. This sub-study is a secondary analysis based on subjects of whom inflammatory biomarkers were available (Fig. 1).
Intervention
Subjects either received the active product or the control product for a double-blind period of 13 weeks, as two 40-g sachets to be dissolved in 125 ml of water and consumed before breakfast and lunch, respectively. Per serving, the active product contained 20 g of whey protein, 3 g total leucine, and a mixture of carbohydrates and fat providing 150 kcal per serving, 800 IU VitD and a mixture of fibres, minerals and vitamins. The isocaloric control product did not contain any protein or micronutrients; detailed compositions are shown in the Supplemental Table of the main PROVIDE publication [21].
Outcome measures
At the baseline visit (within 1 week after the screening visit), characteristics such as age, sex, BMI, ethnicity, living situation, medical history, cognitive function (mini-mental state examination) [24] and pre-existing conditions, use of nutritional supplements and medication were recorded. At baseline and after 7 and 13 weeks, subjects underwent assessments including handgrip strength, body composition, physical performance test and activity, dietary intake and blood sampling as described previously [21]. The European version of the Physical Activity Scale for the Elderly (PASE) was used to assess physical activity. Body composition was measured using dual energy X-ray absorptiometry (DXA, different models from Hologic, Bedford, USA; and Lunar, Fairfield, USA). Dietary VitD and protein intake were assessed by a 3-day dietary record, including two week days and one weekend day. Dietary records were checked for completeness with participants during study visits and additional information was obtained about unclear items or amounts. Total energy, macronutrient and micronutrient intakes were calculated by the participating sites using country-specific dietary data entry systems and food composition tables.
After an overnight fast, serum samples were collected and frozen at – 80 °C until assayed for cytokines levels. IL-8 (ultrasensitive), IL-1RA and sTNFR1 were measured separately using commercially available ELISA kits (Lifetech, Carlsbad, CA), and IL-6 using an ultrasensitive singleplex bead kit (Lifetech, USA) as described previously [25]. Sensitivity levels were < 0.1 pg/ml (IL-8), 4 pg/ml (IL-1RA), 50 pg/ml (sTNFR1) and < 0.05 pg/ml (IL-6). For each participant the samples of both time points were analysed on the same plate to limit inter-assay variability. At baseline and 13 weeks, samples for determination of cytokines were available from respectively 365 and 288 subjects (Fig. 1). Baseline characteristics of subjects with missing data for cytokines were similar to those who were included in the analyses, except for female subjects with missing data in whom the PASE was significantly lower (47.61 ± 31.36 versus 99.96 ± 67.21, p < 0.001).
At baseline, and after 7 and 13 weeks, CRP, pre-albumin and 25-hydroxyvitamin(OH)D were determined by the central PROVIDE laboratory as described elsewhere [21, 22]. For these analyses, several samples were missing at baseline (n = 3 out of 365), 7 weeks (n = 10 out of 288) and 13 weeks (n = 17 out of 288). For 31 (out of 288) participants, no information was available for VitD intake throughout the study.
Statistical analyses
Statistical analyses were performed in IBM SPSS v25.0.0.0 (SPSS Inc, Illinois, USA). Because of non-normal distribution (Kolmogorov–Smirnov Goodness of Fit test p < 0.05), all inflammatory markers were log (10)-transformed to reduce skewness and back transformed for data presentation (Supplementary Table 1). Baseline between group differences were analysed by unpaired t tests. Pearson correlations were computed between baseline inflammatory markers and other baseline outcomes.
Changes in cytokines over time were analysed with repeated-measures ANCOVA using time as within subject’s factor and intervention (active versus control product) group as between subject’s factor. Since dietary intake of VitD and protein might interfere with the nutritional supplement, the mean dietary intake of these components (average of intake measured at baseline, 7 weeks and 13 weeks) were entered in the models as covariates. We also verified whether other relevant factors including fat mass, NSAID use, SPPB, PASE, sex, and baseline 25(OH)D were significant covariates in the analyses. Since only mean dietary VitD intake was a significant factor, this was finally retained as covariate. In addition, these analyses were repeated including only participants with CRP ≤ 10 mg/Llthroughout the entire study to eliminate potential bias due to acute inflammatory conditions (reflected by CRP-value > 10 mg/l [26]).
Next, linear regression was used to appraise the proportional contribution of changes in circulating 25(OH)D and protein (reflected by circulating pre-albumin [27]) to the changes in those cytokines for which a significant time × treatment interaction was found. The change in cytokine level was used as dependent variable and mean dietary intake of VitD and protein, as well as change in pre-albumin and circulating 25(OH)D values were used as predictors. Significance was set at p < 0.05.
Results
There were no significant differences between both groups at baseline (Table 1). Sex differences in body composition and muscle strength were reported previously [21] and were in line with the expectations.
As shown in Table 2, baseline cytokines and dietary protein intake were not significantly correlated. Higher dietary VitD intake and circulatory 25(OH)D were significantly related to lower IL-8. Baseline SPPB and PASE were negatively correlated with baseline cytokines. Fat mass correlated negatively with IL-8 and positively with CRP.
IL-6 and IL-1Ra showed an overall significant increase after 13 weeks (p = 0.006 and p < 0.001, respectively; Fig. 2). For IL-6 a significant time × treatment interaction (p = 0.046) showed that the increase was attenuated in the active (no increase: 1.95 ± 1.09 to 2.17 ± 1.08 pg/ml; p = 0.155) compared to control (significant increase: 1.96 ± 1.09 to 2.56 ± 1.07 pg/ml; p = 0.012) group. IL-8 showed an overall significant decrease (p = 0.03), but there was no significant time × treatment interaction (p = 0.24). Back transformed data on changes over time can be found in Supplementary Table 1.
To eliminate potential bias due to acute inflammatory conditions during the study, we identified all participants who showed a CRP-value > 10 mg/l—as an indicator for an active inflammatory condition [26]—at any time point during the study. In participants with baseline CRP ≤ 10 mg/l, 14 persons acquired an inflammatory profile (i.e. CRP-value > 10 mg/l) after 7 weeks intervention (10 in the active and 4 in the control group, p = 0.057). At 13 weeks, three participants retained an inflammatory profile (two in the active and one in the control group, p = 0.913). For participants showing no inflammation in the previous period, 13 acquired an inflammatory profile at week 13 (6 in the active and 7 in the control group, p = 0.956). When including only participants with CRP ≤ 10 mg/l throughout the entire study (n = 227; active n = 103 and control n = 124; subjects with missing data for CRP were also excluded), comparable results, though more pronounced, were found (see Supplementary Fig. 1).
Finally, we computed a linear regression model to appraise the proportional association of changes in circulating 25(OH)D and pre-albumin to the changes in IL-6 (Table 3). Only the change in pre-albumin was significantly associated with changes in IL-6 after 13 weeks.
Discussion
We assessed the effect of a 13-week VitD and leucine-enriched whey protein oral nutritional intervention on CLIP in sarcopenic older adults with mobility limitations. We found an overall increase in CLIP (demonstrated by IL-6 and IL-1RA), which was significantly attenuated in the active group compared to control for IL-6. When excluding participants who might have experienced pathologic acute inflammation during the study (based on CRP-values > 10 mg/l), the anti-inflammatory effects of the active intervention remained significant, and was even more pronounced.
CLIP is a well-known phenomenon in the aged [1, 28,29,30]. Contributing factors include immunosenescence, lack of physical activity, decline of sex hormones and increase in adipose tissue [2, 31,32,33,34]. The exact kinetics of CLIP are not well described in the literature. However, CLIP is more pronounced in (pre)frail older adults and/or older subjects with chronic diseases [1, 4, 35,36,37]. Given the specific profile of our participants with low muscle mass and mobility limitations, it can be expected that CLIP would have progressed more rapidly than expected in a group of healthier older persons. The IL-6 levels in our participants (median = 1.97 pg/ml, P25–P75 = [1.19–2.96]) correspond to the levels for CLIP (2.13 pg/ml [1.37–4.23]) as recently reviewed [30]. The cross-sectional data reviewed by Calder et al. [30] suggest mean differences of about 0.7 pg/ml for log IL-6 between young and older adults. In this context the difference in change in IL-6 between active and control group that we observed might be of clinical relevance.
Our findings are in line with a recently published RCT investigating the additional effect of a combined VitD and whey protein oral nutritional supplement on exercise-induced changes in CRP in sarcopenic older persons [38]. They reported a significant time × treatment effect after 12 weeks intervention (p = 0.04) characterized by a tendency for CRP to increase in the placebo group (+ 4.4 mg/l, p = 0.06) which was attenuated in the intervention group (− 1.9 mg/l, p = 0.33) [38]. However, these authors did not quantify other biomarkers of CLIP.
VitD and protein supplementation can reduce CLIP through several pathways. VitD inhibits T-cell proliferation, inhibiting Th1 and Th17 pro-inflammatory responses and stimulating Th2 response, resulting in a decreased inflammatory profile [15, 39]. Inflammatory cells can convert VitD into calcitriol, which can itself regulate the cytokines by blocking NF-kß p65 activation (by upregulation of ikBa) which in its turn can inhibit differentiation of B-cells to plasma cells [40]. In dendritic cells, VitD3 downregulates the expression of pro-inflammatory cytokines, inhibits differentiation of plasma cells and upregulates expression of anti-inflammatory cytokines (such as IL-10) as well as inflammation-inhibiting molecules such as ILT-3 [41]. Dietary proteins are crucial for muscle anabolism and considered to play a major role in countering sarcopenia and CLIP [42]. Amino acids such as leucine have strong anabolic effects and stimulate intramuscular protein synthesis via upregulation of the mTOR pathway, which also reduces protein breakdown and can induce anti-inflammatory effects [8, 43]. Also β-hydroxy β-methylbutyrate, a metabolite of leucine, has anti-inflammatory effects in older persons [44].
As both VitD and protein—as main components of the nutritional supplement—can have anti-inflammatory effects, linear regression analysis was used to appraise the proportional contribution of changes in circulating 25(OH)D and protein (reflected by circulating pre-albumin) to the changes in IL-6. Our results point towards the effects of the protein component of the nutritional supplementation (reflected by the changes in circulating pre-albumin) as significantly associated with the attenuation in IL-6 increase. Although many studies investigated the effect of nutritional supplementation on physical outcomes, very few investigated the effects on inflammation in older adults [45, 46]. Most studies did not find any effect of VitD on inflammation [47, 48]. Studies reporting significant effects were contradictory and remained inconclusive (see Ref. [49] for review).
Our findings regarding IL-8 are in line with those reported by Yusupov et al. who found that IL-8 decreased significantly after 12 weeks of VitD supplementation (− 48%, p < 0.001), which was comparable to the decrease in the control group (− 33%, p = 0.02) [50]. Nakashyan et al. recently performed a study on constitutive and IL-1ß-stimulated human gingival fibroblast, where they analysed the effect of VitD on levels of IL-6. In constitutive fibroblast, IL-6 production decreased by ~ 50%. When cells were stimulated with IL-1ß, the effects on IL-6 depended on the time when the cells were exposed to VitD. The longer the time in between the IL-1ß stimulus and the VitD exposure, the smaller the effect observed [51]. Considering the inflammatory effects of IL-1ß, this is comparable to the results obtained in our analyses, where a time effect was seen when all participants were included. When participants who acquired an inflammatory profile (i.e. CRP > 10 mg/l) were excluded from our analyses, the effects on IL-6 were more pronounced. In our study, the levels of circulating sTNFR1 were not related to protein or VitD, and did not show changes over time. Similarly, Vita et al. found no correlation between VitD and sTNFR1 or IL-1Ra in older adults [14].
The strengths of this study are the double-blinded randomized and controlled design and the large sample size. Also, the analyses with high-sensitivity ELISA kits allowed to detect small changes in inflammatory markers. However, the results should be interpreted cautiously, since the nutritional supplement contained other compounds besides VitD, leucine and whey proteins which might have influenced the anti-inflammatory effects. As reported earlier [21, 22], the active product contained micronutrients, including also 2.2 g zinc per serving whereas the iso-caloric control product contained only carbohydrates and fat. Dietary zinc intake as well as circulating zinc levels are inversely related to IL-6, TNF-α and CRP [52]. In addition, Bao et al. found that IL-6 decreased significantly after 6 months of zinc supplementation in 40 older adults [53]. Unfortunately, we have no data regarding zinc status in our participants. Another potential limitation is that the dose of the oral nutritional supplement might not have been sufficiently high to obtain optimal effects. In the study performed by Azizieh et al. participants with deficient VitD levels and high CRP showed higher levels of pro-inflammatory cytokines compared to participants with sufficient VitD levels [54]. In our study, the supplementation dose was not tailored according to, e.g. baseline 25(OH)D status or nutritional protein intake. On the other hand, we used dietary VitD intake as a covariable in our analyses, and the influence of other potential confounders such as dietary protein intake, physical functioning, physical activity and NSAID use has been verified. In this study we used pre-albumin level as a surrogate marker for the protein-related nutritional status. However, since pre-albumin level can—at least in acute inflammatory conditions—be influenced by ongoing inflammation [55, 56], we cannot exclude that this might have affected our results. Therefore, the role of the proteins in the supplement on the attenuation of CLIP in our study needs to be interpreted with caution. 25% of the randomized subjects were lost to follow-up or showed missing data, which is relatively high but acceptable for a clinical trial in older patients with mobility limitations. Finally, as this was a multi-center study, environmental factors such as season of the year or exposure to sunlight—which is a source of VitD—might have influenced the results [57].
Conclusions
Based on the results of our study, we conclude that 13 weeks of nutritional supplementation with VitD and leucine-enriched whey protein may attenuate the progression of chronic low-grade inflammation in older sarcopenic persons with mobility limitations. Our results can be of clinical significance since chronic low-grade inflammation is a major contributor to the progression of frailty and sarcopenia.
References
Krabbe K, Pedersen M, Bruunsgaard H (2004) Inflammatory mediators in the elderly. Exp Gerontol 39:687–699
Beyer I, Mets T, Bautmans I (2012) Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care 15:12–22
Soysal P et al (2016) Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 31:1–8
Cao Dinh H et al (2019) Association between immunosenescence phenotypes and pre-frailty in older subjects: does cytomegalovirus play a role? J Gerontol Ser A Biol Sci Med Sci 74:480–488
Vermeiren S et al (2016) Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 17:1163
Cruz-Jentoft AJ et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Boirie Y (2013) Fighting sarcopenia in older frail subjects: protein fuel for strength, exercise for mass. J Am Med Dir Assoc 14:140–143
Xia Z et al (2017) Targeting inflammation and downstream protein metabolism in sarcopenia: a brief up-dated description of concurrent exercise and leucine-based multimodal intervention. Front Physiol 8:434
Kato H et al (2016) Leucine-enriched essential amino acids attenuate inflammation in rat muscle and enhance muscle repair after eccentric contraction. Amino Acids 48:2145–2155
Breitman I et al (2011) The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. J Am Coll Surg 212:617–625 (discussion 625–627)
Zhenyukh O et al (2017) High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 104:165–177
Bonvini A et al (2018) Immunomodulatory role of branched-chain amino acids. Nutr Rev 76:840–856
Bauer J et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14:542–559
De Vita F et al (2014) Relationship between vitamin D and inflammatory markers in older individuals. Age (Dordr) 36:9694
Guillot X et al (2010) Vitamin D and inflammation. Jt Bone Spine 77:552–557
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Manios Y et al (2018) Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: the Food4Me study. Eur J Nutr 57:1257
Barnes MS et al (2011) Maintenance of wintertime vitamin D status with cholecalciferol supplementation is not associated with alterations in serum cytokine concentrations among apparently healthy younger or older adults. J Nutr 141:476–481
Wood AD et al (2012) Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 97:3557–3568
Wamberg L et al (2013) Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—results from a randomized trial. Eur J Intern Med 24:644–649
Bauer JM et al (2015) Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 16:740–747
Verlaan S et al (2018) Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults—The PROVIDE study. Clin Nutr 37:551–557
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Forti LN et al (2016) Load-specific inflammation mediating effects of resistance training in older persons. J Am Med Dir Assoc 17:547–552
Martinez VB, Gonzalez-Juanatey JR (2009) Markers of inflammation and cardiovascular disease: clinical applications of C-reactive protein determination. Am J Cardiovasc Drugs 9:3–7
Beck FK, Rosenthal TC (2002) Prealbumin: a marker for nutritional evaluation. Am Fam Phys 65:1575–1578
Wei J et al (1992) Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci 51:1953–1956
Ershler WB et al (1993) Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res 12:225–230
Calder PC et al (2017) Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev 40:95–119
Singh T, Newman AB (2011) Inflammatory markers in population studies of aging. Ageing Res Rev 10:319–329
Pedersen BK (2009) The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol 587:5559–5568
Fulop T et al (2017) Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 8:1960
Pawelec G, Goldeck D, Derhovanessian E (2014) Inflammation, ageing and chronic disease. Curr Opin Immunol 29:23–28
Baggio G et al (1998) Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J 12:433–437
Franceschi C et al (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105
Bruunsgaard H, Pedersen BK (2003) Age-related inflammatory cytokines and disease. Immunol Allergy Clin N Am 23:15–39
Rondanelli M et al (2016) Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 103:830–840
Boonstra A et al (2001) 1alpha, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167:4974–4980
Colotta F, Jansson B, Bonelli F (2017) Modulation of inflammatory and immune responses by vitamin D. J Autoimmun 85:78–97
Vanherwegen AS, Gysemans C, Mathieu C (2017) Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol Cell Endocrinol 453:52–67
Paddon-Jones D et al (2015) Protein and healthy aging. Am J Clin Nutr 101:1339S–1345S
Crozier SJ et al (2005) Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135:376–382
Arazi H, Taati B, Suzuki K (2018) A review of the effects of leucine metabolite (beta-hydroxy-beta-methylbutyrate) supplementation and resistance training on inflammatory markers: a new approach to oxidative stress and cardiovascular risk factors. Antioxidants (Basel) 7:148
Ticinesi A et al (2016) Nutrition and inflammation in older individuals: focus on vitamin D, n-3 polyunsaturated fatty acids and whey proteins. Nutrients 8:186
Calton EK et al (2017) The impact of cholecalciferol supplementation on the systemic inflammatory profile: a systematic review and meta-analysis of high-quality randomized controlled trials. Eur J Clin Nutr 71:931–943
Agbalalah T et al (2017) Impact of vitamin D supplementation on endothelial and inflammatory markers in adults: a systematic review. J Steroid Biochem Mol Biol 173:292–300
Jamka M et al (2016) The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr 55:2163–2176
Goncalves de Carvalho CM, Ribeiro SM (2017) Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr 71:434–440
Yusupov E et al (2010) Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol 2010:305054
Nakashyan V et al (2017) Effect of 1,25(OH)2 D3 and 20(OH)D3 on interleukin-1beta-stimulated interleukin-6 and -8 production by human gingival fibroblasts. J Periodontal Res 52:832–841
Jung S, Kim MK, Choi BY (2015) The relationship between zinc status and inflammatory marker levels in rural Korean adults aged 40 and older. PLoS One 10:e0130016
Bao B et al (2010) Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr 91:1634–1641
Azizieh F, Alyahya KO, Raghupathy R (2016) Association between levels of vitamin D and inflammatory markers in healthy women. J Inflamm Res 9:51–57
Nouvenne A et al (2016) The prognostic value of high-sensitivity C-reactive protein and prealbumin for short-term mortality in acutely hospitalized multimorbid elderly patients: a prospective cohort study. J Nutr Health Aging 20:462–468
Dennis RA et al (2008) Changes in prealbumin, nutrient intake, and systemic inflammation in elderly recuperative care patients. J Am Geriatr Soc 56:1270–1275
Wacker M, Holick MF (2013) Sunlight and vitamin D: a global perspective for health. Dermato-endocrinology 5:51–108
Acknowledgements
We thank the participants for giving their time and energy to volunteer for this trial. We are grateful to the research staff members at each study site for their energy and commitment to achieving the aims of this study. We thank Dr. Wilfried Cools ("Interfaculty Center Data processing and Statistics (ICDS), Vrije Universiteit Brussel) for the bio-statistical advice. We are thankful to the PROVIDE study group* for their professional oversight and guidance. This study was financially supported and study products were provided by Nutricia Research, Nutricia Advanced Medical Nutrition. *PROVIDE study group members: Jürgen M. Bauer, Sjors Verlaan, Ivan Bautmans, Kirsten Brandt, Lorenzo M. Donini, Marcello Maggio, Marion E. T. McMurdo, Tony Mets, Chris Seal, Sander L. Wijers, Gian Paolo Ceda, Giuseppe De Vito, Gilbert Donders, Michael Drey, Carolyn Greig, Ulf Holmbäck, Marco Narici, Jamie McPhee, Eleonora Poggiogalle, Dermot Power, Aldo Scafoglieri, Ralf Schultz, Cornel C. Sieber, Tommy Cederholm.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tommy Cederholm has given remunerated lectures and has received unconditional research grants from Nutricia, Nestle. Sander Wijers and Yvette Luiking are employed by Nutricia Research at the Nutricia advanced medical nutrition department. Jürgen Bauer is scientific advisor, has received research Grants and has received speakers honorarium from Nutricia, Nestlé. Keliane Liberman, Ivan Bautmans, Tony Mets, Rose Njemini, Lorenzo M Donini, Marcello Giuseppe Maggio, Kirsten Brandt, Louis Forti, Cornel Sieber, Robert Memelink and Sjors Verlaan declare that they have no conflict of interest.
Human and animal rights
The study protocol was approved by institutional review boards at each location and registered under the Dutch trials register with the identifier: NTR2329 (http://www.trialregister.nl/trialreg). Study procedures were performed in accordance with the Declaration of Helsinki ethical principles for medical research involving human subjects.
Informed consent
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liberman, K., Njemini, R., Luiking, Y. et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res 31, 845–854 (2019). https://doi.org/10.1007/s40520-019-01208-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01208-4