Abstract
Background
Quantitative analysis of mitochondrial morphology plays important roles in studies of mitochondrial biology. The analysis depends critically on segmentation of mitochondria, the image analysis process of extracting mitochondrial morphology from images. The main goal of this study is to characterize the performance of convolutional neural networks (CNNs) in segmentation of mitochondria from fluorescence microscopy images. Recently, CNNs have achieved remarkable success in challenging image segmentation tasks in several disciplines. So far, however, our knowledge of their performance in segmenting biological images remains limited. In particular, we know little about their robustness, which defines their capability of segmenting biological images of different conditions, and their sensitivity, which defines their capability of detecting subtle morphological changes of biological objects.
Methods
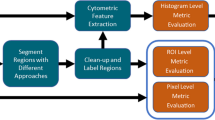
We have developed a method that uses realistic synthetic images of different conditions to characterize the robustness and sensitivity of CNNs in segmentation of mitochondria. Using this method, we compared performance of two widely adopted CNNs: the fully convolutional network (FCN) and the U-Net. We further compared the two networks against the adaptive active-mask (AAM) algorithm, a representative of high-performance conventional segmentation algorithms.
Results
The FCN and the U-Net consistently outperformed the AAM in accuracy, robustness, and sensitivity, often by a significant margin. The U-Net provided overall the best performance.
Conclusions
Our study demonstrates superior performance of the U-Net and the FCN in segmentation of mitochondria. It also provides quantitative measurements of the robustness and sensitivity of these networks that are essential to their applications in quantitative analysis of mitochondrial morphology.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
McBride, H. M., Neuspiel, M. and Wasiak, S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol., 16, R551–R560
Nunnari, J. and Suomalainen, A. (2012) Mitochondria: in sickness and in health. Cell, 148, 1145–1159
Karbowski, M. and Youle, R. J. (2003) Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ., 10, 870–880
Campello, S. and Scorrano, L. (2010) Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep., 11, 678–684
Chen, K. C. J., Yu, Y. Y., Li, R. Q., Lee, H. C., Yang, G. and Kovacevic, J. (2012) Adaptive active-mask image segmentation for quantitative characterization of mitochondrial morphology. In Proceedings of 2012 IEEE International Conference on Image Processing (ICIP), pp. 2033–2036
Leonard, A. P., Cameron, R. B., Speiser, J. L., Wolf, B. J., Peterson, Y. K., Schnellmann, R. G., Beeson, C. C. and Rohrer, B. (2015) Quantitative analysis of mitochondrial morphology and membrane potential in living cells using high-content imaging, machine learning, and morphological binning. Biochim. Biophys. Acta, 1853, 348–360
Peng, J.-Y., Lin, C.-C., Chen, Y.-J., Kao, L.-S., Liu, Y.-C., Chou, C.-C., Huang, Y.-H., Chang, F.-R., Wu, Y.-C., Tsai, Y.-S., et al. (2011) Automatic morphological subtyping reveals new roles of caspases in mitochondrial dynamics. PLoS Comput. Biol., 7, e1002212
Iannetti, E. F., Smeitink, J. A. M., Beyrath, J., Willems, P. H. G. M. and Koopman, W. J. H. (2016) Multiplexed high-content analysis of mitochondrial morphofunction using live-cell microscopy. Nat. Protoc., 11, 1693–1710
Daniele, J. R., Esping, D. J., Garcia, G., Parsons, L. S., Arriaga, E. A. and Dillin, A. (2017) High-throughput characterization of region-specific mitochondrial function and morphology. Sci. Rep., 7, 6749
Krizhevsky, A., Sutskever, I. and Hinton, G. E. (2012) ImageNet classification with deep convolutional neural networks. In Proceedings of the 25th International Conference on Neural Information Processing Systems. pp. 1097–1105
LeCun, Y., Bengio, Y. and Hinton, G. (2015) Deep learning. Nature, 521, 436–444
Sadanandan, S. K., Ranefall, P., Le Guyader, S. and Wählby, C. (2017) Automated training of deep convolutional neural networks for cell segmentation. Sci. Rep., 7, 7860
Kraus, O. Z., Ba, J. L. and Frey, B. J. (2016) Classifying and segmenting microscopy images with deep multiple instance learning. Bioinformatics, 32, i52–i59
Xing, F., Xie, Y., Su, H., Liu, F. and Yang, L. (2017) Deep learning in microscopy image analysis: a survey. In IEEE Transactions on Neural Networks and Learning Systems. pp. 1–19
Yu, Y., Lee, H.-C., Chen, K.-C., Suhan, J., Qiu, M., Ba, Q. and Yang, G. (2016) Inner membrane fusion mediates spatial distribution of axonal mitochondria. Sci. Rep., 6, 18981
Long, J., Shelhamer, E. and Darrell, T. (2015) Fully convolutional networks for semantic segmentation. In 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). pp. 3431–3440
Ronneberger, O., Fischer, P. and Brox, T. (2015) U-Net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention. 9351, 234–241
Lehmussola, A., Ruusuvuori, P., Selinummi, J., Huttunen, H. and Yli-Harja, O. (2007) Computational framework for simulating fluorescence microscope images with cell populations. IEEE Trans. Med. Imaging, 26, 1010–1016
Li, C., Huang, R., Ding, Z., Gatenby, J. C., Metaxas, D. N. and Gore, J. C. (2011) A level set method for image segmentation in the presence of intensity inhomogeneities with application to MRI. IEEE Trans. Image Process., 20, 2007–2016
Ngo, T. A., Lu, Z. and Carneiro, G. (2017) Combining deep learning and level set for the automated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance. Med. Image Anal., 35, 159–171
Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675
Abadi, M., Barham, P., Chen, J., Chen, Z., Davis, A., Dean, J., Devin, M., Ghemawat, S., Irving, G., Isard, M., et al. (2016) TensorFlow: a system for large-scale machine learning. In Proceedings of the 12th USENIX Conference on Operating Systems Design and Implementation. pp. 265–283
Heimann, T., van Ginneken, B., Styner, M. A., Arzhaeva, Y., Aurich, V., Bauer, C., Beck, A., Becker, C., Beichel, R., Bekes, G., et al. (2009) Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE Trans. Med. Imaging, 28, 1251–1265
Acknowledgements
Xiaoqi Chai acknowledges support of a Ji-Dian Liang Graduate Research Fellowship. Qinle Ba acknowledges support of a Bertucci Graduate Research Fellowship. Ge Yang acknowledges support of NSF CAREER grant DBI-1149494 and NSF grant CBET-1804929. The authors would also like to thank Yile Feng and Weicheng Lin for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author summary: Segmentation of mitochondria, the image analysis process of extracting geometry of mitochondria from their images, plays an important role in elucidating their biology. Convolutional neural networks (CNNs), artificial neural networks widely used in artificial intelligence, have achieved great success in segmenting mitochondria. However, little is known about their robustness in segmenting mitochondria under different image conditions and their sensitivity in detecting subtle mitochondrial shape changes. Here we develop a method of using synthesized images to characterize performance of CNNs, specifically FCN and U-Net. Our study demonstrates their superior performance in segmentation of mitochondria and directly quantifies their robustness and sensitivity.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chai, X., Ba, Q. & Yang, G. Characterizing robustness and sensitivity of convolutional neural networks for quantitative analysis of mitochondrial morphology. Quant Biol 6, 344–358 (2018). https://doi.org/10.1007/s40484-018-0156-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40484-018-0156-3