Abstract
Background
Informal care makes an important contribution to societal welfare. However, it may involve substantial time costs and can have a considerable negative effect on the health and well-being of informal caregivers. These costs and effects of informal caregiving are often excluded in economic evaluations of healthcare interventions. The impact of this exclusion on the outcomes of these evaluations is largely unknown.
Objectives
This study aimed to explore the inclusion of informal care in economic evaluations and the potential impact of the costs and effects of informal caregiving on cost-effectiveness outcomes.
Methods
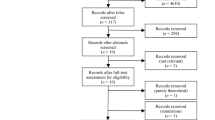
A systematic review was conducted to identify economic evaluations of interventions in four distinct disease areas where informal care is potentially important: Alzheimer’s disease, metastatic colorectal cancer, Parkinson’s disease and rheumatoid arthritis. It was recorded how often economic evaluations included informal caregiving. Next, for the studies including informal care, the impact on cost-effectiveness outcomes was determined by removing informal care costs and effects of the cost-effectiveness calculations and recalculating the outcomes. The new cost-effectiveness outcomes were then compared with the original reported outcomes.
Results
The study identified 100 economic evaluations investigating interventions targeted at Alzheimer’s disease (n = 25), metastatic colorectal cancer (n = 24), Parkinson’s disease (n = 8) and rheumatoid arthritis (n = 43). Twenty-three of these evaluations (23 %) included costs and/or effects of informal caregiving: 64 % of the Alzheimer’s disease studies, 0 % of the metastatic colorectal cancer studies, 13 % of Parkinson’s disease studies and 14 % of rheumatoid arthritis studies. When informal care was included, this mostly concerned time costs. Studies rarely included both costs and effects. The effect of including or excluding informal care costs or effects on cost-effectiveness outcomes in most studies was modest, but in some studies the impact was strong.
Conclusion
Most economic evaluations in the area of Alzheimer’s disease include costs and/or effects related to informal caregiving. However, in other disease areas where informal caregiving is common it seems that the majority of economic evaluations ignore informal caregiving. The inclusion of informal care can have a strong impact on cost-effectiveness outcomes. Future economic evaluations should therefore consider the relevance of informal care in the context of their study, and either include these costs and effects or justify why they were excluded.


Similar content being viewed by others
References
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford/New York: Oxford University Press; 2005.
Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ. 2005;24:751–73.
Christakis NA. Social networks and collateral health effects. BMJ. 2004;329:184–5.
Bobinac A, van Exel NJ, Rutten FF, Brouwer WB. Caring for and caring about: disentangling the caregiver effect and the family effect. J Health Econ. 2010;29:549–56.
Bobinac A, van Exel NJ, Rutten FF, Brouwer WB. Health effects in significant others: separating family and care-giving effects. Med Decis Making. 2011;31:292–8.
Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. Pharmacoeconomics. 2013;31:1105–19.
Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–9.
Pitsenberger DJ. Juggling work and elder caregiving: work-life balance for aging American workers. AAOHN J. 2006;54:181–5 (quiz 186–7).
van Ryn M, Sanders S, Kahn K, van Houtven C, Griffin JM, Martin M, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology. 2011;20:44–52.
Brouwer WB. Too important to ignore: informal caregivers and other significant others. Pharmacoeconomics. 2006;24:39–41.
Dixon S, Walker M, Salek S. Incorporating carer effects into economic evaluation. Pharmacoeconomics. 2006;24:43–53.
Davidson T, Levin LA. Is the societal approach wide enough to include relatives? Incorporating relatives’ costs and effects in a cost-effectiveness analysis. Appl Health Econ Health Policy. 2010;8:25–35.
Al-Janabi H, Flynn TN, Coast J. QALYs and carers. Pharmacoeconomics. 2011;29:1015–23.
Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26:269–80.
Goodrich K, Kaambwa B, Al-Janabi H. The inclusion of informal care in applied economic evaluation: a review. Value Health. 2012;15:975–81.
Opara JA. Activities of daily living and quality of life in Alzheimer disease. J Med Life. 2012;5:162–7.
Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12:35–41.
Sansoni J, Anderson KH, Varona LM, Varela G. Caregivers of Alzheimer’s patients and factors influencing institutionalization of loved ones: some considerations on existing literature. Ann Ig. 2013;25:235–46.
Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26:77–90.
Brouwer WB, van Exel NJ, van de Berg B, Dinant HJ, Koopmanschap MA, van den Bos GA. Burden of caregiving: evidence of objective burden, subjective burden, and quality of life impacts on informal caregivers of patients with rheumatoid arthritis. Arthritis Rheum. 2004;51:570–7.
Dalal KM, Gollub MJ, Miner TJ, Wong WD, Gerdes H, Schattner MA, et al. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. J Palliat Med. 2011;14:822–8.
Gao W, Gulliford M, Bennett MI, Murtagh FE, Higginson IJ. Managing cancer pain at the end of life with multiple strong opioids: a population-based retrospective cohort study in primary care. PLoS One. 2014;9:e79266.
Stone PW, Chapman RH, Sandberg EA, Liljas B, Neumann PJ. Measuring costs in cost-utility analyses. Variations in the literature. Int J Technol Assess Health Care. 2000;16:111–24.
Hoefman RJ, Van Exel NJA, Brouwer WBF. iMTA Valuation of Informal Care Questionnaire (iVICQ). Version 1.0 (December 2011). Rotterdam: iBMG/iMTA; 2011. http://www.bmg.eur.nl/english/imta/publications/manuals_questionnaires/#iVICQ. Accessed 12 Apr 2014.
Rive B, Aarsland D, Grishchenko M, Cochran J, Lamure M, Toumi M. Cost-effectiveness of memantine in moderate and severe Alzheimer’s disease in Norway. Int J Geriatr Psychiatry. 2012;27:573–82.
Meeuwsen E, Melis R, van der Aa G, Goluke-Willemse G, de Leest B, van Raak F, et al. Cost-effectiveness of one year dementia follow-up care by memory clinics or general practitioners: economic evaluation of a randomised controlled trial. PLoS One. 2013;8:e79797.
Touchon J, Lachaine J, Beauchemin C, Granghaud A, Rive B, Bineau S. The impact of memantine in combination with acetylcholinesterase inhibitors on admission of patients with Alzheimer’s disease to nursing homes: cost-effectiveness analysis in France. Eur J Health Econ. Epub 2013 Aug 9.
Getsios D, Blume S, Ishak KJ, Maclaine G, Hernandez L. An economic evaluation of early assessment for Alzheimer’s disease in the United Kingdom. Alzheimers Dement. 2012;8:22–30.
Pfeil AM, Kressig RW, Szucs TD. Alzheimer’s dementia: budget impact and cost-utility analysis of a combination treatment of a cholinesterase inhibitor and memantine in Switzerland. Swiss Med Wkly. 2012;142:w13676.
López-Bastida J, Hart W, Garcia-Perez L, Linertova R. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2009;16:399–407.
Zhang Y, Kivipelto M, Solomon A, Wimo A. Cost-effectiveness of a health intervention program with risk reductions for getting demented: results of a Markov model in a Swedish/Finnish setting. J Alzheimers Dis. 2011;26:735–44.
Hoogveldt B, Rive B, Severens J, Maman K, Guilhaume C. Cost-effectiveness analysis of memantine for moderate-to-severe Alzheimer’s disease in The Netherlands. Neuropsychiatr Dis Treat. 2011;7:313–7.
Biasutti M, Dufour N, Ferroud C, Dab W, Temime L. Cost-effectiveness of magnetic resonance imaging with a new contrast agent for the early diagnosis of Alzheimer’s disease. PLoS One. 2012;7:e35559.
van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, de Vries-Bouwstra JK, Hazes JM, Kerstens PJ, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2009;61:291–9.
Lekander I, Borgstrom F, Svarvar P, Ljung T, Carli C, van Vollenhoven RF. Cost-effectiveness of real-world infliximab use in patients with rheumatoid arthritis in Sweden. Int J Technol Assess Health Care. 2010;26:54–61.
Ungar WJ, Costa V, Hancock-Howard R, Feldman BM, Laxer RM. Cost-effectiveness of biologics in polyarticular-course juvenile idiopathic arthritis patients unresponsive to disease-modifying antirheumatic drugs. Arthritis Care Res. 2011;63:111–9.
Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost-effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. Int J Technol Assess Health Care. 2011;27:193–200.
Lekander I, Borgstrom F, Lysholm J, van Vollenhoven RF, Lindblad S, Geborek P, et al. The cost-effectiveness of TNF-inhibitors for the treatment of rheumatoid arthritis in Swedish clinical practice. Eur J Health Econ. 2013;14:863–73.
Lindgren P, Geborek P, Kobelt G. Modeling the cost-effectiveness of treatment of rheumatoid arthritis with rituximab using registry data from Southern Sweden. Int J Technol Assess Health Care. 2009;25:181–9.
Hartz S, Getsios D, Tao S, Blume S, Maclaine G. Evaluating the cost effectiveness of donepezil in the treatment of Alzheimer’s disease in Germany using discrete event simulation. BMC Neurol. 2012;12:2.
Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal no. 111): a systematic review and economic model. Health Technol Assess. 2012;16:1–470.
Woods RT, Bruce E, Edwards RT, Elvish R, Hoare Z, Hounsome B, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers—effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol. Assess. 2012;16:v–xv (1–116).
Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial—a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess. 2013;17:1–166.
Nagy B, Brennan A, Brandtmuller A, Thomas SK, Sullivan SD, Akehurst R. Assessing the cost-effectiveness of the rivastigmine transdermal patch for Alzheimer’s disease in the UK using MMSE- and ADL-based models. Int J Geriatr Psychiatry. 2011;26:483–94.
Romeo R, Knapp M, Hellier J, Dewey M, Ballard C, Baldwin R, et al. Cost-effectiveness analyses for mirtazapine and sertraline in dementia: randomised controlled trial. Br J Psychiatry. 2013;202:121–8.
Haycox A, Armand C, Murteira S, Cochran J, Francois C. Cost effectiveness of rasagiline and pramipexole as treatment strategies in early Parkinson’s disease in the UK setting: an economic Markov model evaluation. Drugs Aging. 2009;26:791–801.
Suh GH. Modeling the cost-effectiveness of galantamine for mild to moderately severe Alzheimer’s disease in Korea. Value Health. 2009;12:S49–54.
Acknowledgments
This study is part of a larger project investigating the broader societal benefits of healthcare, which was financially supported by AstraZeneca, GlaxoSmithKline, Janssen, MSD BV, Novartis and Pfizer. The views expressed in this paper are those of the authors. The authors have no conflicts of interest to declare.
Author contributions
MK was primarily responsible for writing the manuscript in close cooperation with JP and JvE. The search strategies conducted in the review were the result of joint effort. MK and JP conducted the actual review process. All authors read, edited and approved the final manuscript. MK is the overall guarantor for the content.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krol, M., Papenburg, J. & van Exel, J. Does Including Informal Care in Economic Evaluations Matter? A Systematic Review of Inclusion and Impact of Informal Care in Cost-Effectiveness Studies. PharmacoEconomics 33, 123–135 (2015). https://doi.org/10.1007/s40273-014-0218-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0218-y