Abstract
Background and Objective
Formative research studies can inform stated-preference instrument development to quantify the importance of various attributes of healthcare treatments. The objective of this study was to elicit from patients with chronic obstructive pulmonary disease the prioritization of an established set of patient-informed value elements.
Methods
Using an iterative mixed-methods study design, we engaged individuals living with chronic obstructive pulmonary disease in Phase 1 value element elicitation and Phase 2 language refinement. Study participants were recruited from March to July 2019. Four guided activities, administered in an online instrument, elicited individual preferences for 40 disease-agnostic value elements that were aligned with treatment, outcomes, or care process. Responses from the guided activities were summarized and then presented to a patient advocate and additional patient participants for further refinement of the value elements and the phrasing.
Results
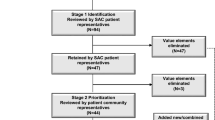
Twenty-three participants, 18 male and five female, mean age of 66 years (standard deviation = 7) were enrolled in Phase 1. Participant responses informed the selection of eight elements as the key candidates for the Phase 2 language refinement: Side Effects, New Therapeutic Option, Available Treatment, Appropriateness of Care, Predictable Healthcare Needs, Physical Activities: Endurance and Symptom Control, and Explanation of Treatment. With feedback from a patient advocate and additional patient participants, elements were refined, rephrased, or modified and this list was narrowed to six value elements (Side Effects, New Therapeutic Option, Willingness to Pay, Physical Activities, Explanation of Treatment, and Access to Care) to serve as attributes in a conceptual framework for a future quantitative stated-preference instrument.
Conclusions
This patient-engaged formative work identified patients with chronic obstructive pulmonary disease key attributes of value-based decision making that underpin benefit-risk trade-offs between physical endurance, treatment side effects, care access, and cost. This study illustrates an iterative process for eliciting and refining a comprehensive list of value elements, resulting in a subgroup of elements important to a specific patient population.

Similar content being viewed by others
Change history
22 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40271-021-00499-y
References
Lortet-Tieulent J, Soerjomataram I, López-Campos JL, Coebergh JW, Ancochea J, Soriano JB. International trends in COPD mortality, 1995–2017. Eur Respir J. 2019;54(6):1901791. https://doi.org/10.1183/13993003.01791-2019.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2015. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf. Accessed 11 Jan 2020.
Zafari Z, Bryan S, Sin DD, Conte T, Khakban R, Sadatsafavi M. A systematic review of health economics simulation models of chronic obstructive pulmonary disease. Value Health. 2017;20(1):152–62. https://doi.org/10.1016/j.jval.2016.08.003.
Gray C, Slejko JF. Identifying COPD patient-informed value elements in economic evaluations: a systematic review. Value Health. 2019;22:S361. https://doi.org/10.1016/j.jval.2019.04.1767.
Bereza BG, Nielsen AT, Valgardsson S, Hemels MEH, Einarson TR. Patient preferences in severe COPD and asthma: a comprehensive literature review. Int J Chron Obstruct Pulmon Dis. 2015;10:739–44. https://doi.org/10.2147/COPD.S82179.
Zhang Y, Morgan RL, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52(1):1800222. https://doi.org/10.1183/13993003.00222-2018.
Cook NS, Cave J, Holtorf A-P. Patient preference studies during early drug development: aligning stakeholders to ensure development plans meet patient needs. Front Med (Lausanne). 2019;6:82. https://doi.org/10.3389/fmed.2019.00082.
Cook N, Gey J, Oezel B, et al. Impact of cough and mucus on COPD patients: primary insights from an exploratory study with an online patient community. Int J Chron Obstruct Pulmon Dis. 2019;4:1365–76. https://doi.org/10.2147/COPD.S202580.
dosReis S, Butler B, Caicedo J, et al. Stakeholder-engaged derivation of patient-informed value elements. Patient. 2020;13(5):611–21. https://doi.org/10.1007/s40271-020-00433-8.
Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy. 2007;12(1):25–30. https://doi.org/10.1258/135581907779497602.
Hollin IL, Craig BM, Coast J, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. 2020;13(1):121–36. https://doi.org/10.1007/s40271-019-00401-x.
Coast J, Al-Janabi H, Sutton EJ, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–41. https://doi.org/10.1002/hec.1739.
dosReis S, Castillo WC, Ross M, Fitz-Randolph M, Vaughn-Lee A, Butler B. Attribute development using continuous stakeholder engagement to prioritize treatment decisions: a framework for patient-centered research. Value Health. 2016;19(6):758–66. https://doi.org/10.1016/j.jval.2016.02.013.
Slejko JF, Gray C, Hong Y, Rueda JD, Zhang C, DosReis S. Aligning COPD outcomes with patient-informed value element domains for use in economic evaluations. Value Health. 2019;22:S351. https://doi.org/10.1016/j.jval.2019.04.1716.
COPD Foundation. COPD online support community: COPD360social. 2020. https://www.copdfoundation.org/COPD360social/Community/Get-Involved.aspx?gclid=CjwKCAjw8-78BRA0EiwAFUw8LHhXrw7kDM-ge5QniFsyd6JbDVk6LI4T46Zp9a8XH__i0xCWdOQdZRoCauIQAvD_BwE. Accessed 11 Jan 2020.
GlaxoSmithKline. The COPD Assessment Test (CAT). 2018. www.CATestonline.org. Accessed 11 Jan 2020.
Svedsater H, Leather D, Robinson T, Doll H, Nafees B, Bradshaw L. Evaluation and quantification of treatment preferences for patients with asthma or COPD using discrete choice experiment surveys. Respir Med. 2017;132:76–83. https://doi.org/10.1016/j.rmed.2017.09.010.
Goossens LM, Rutten-Van Mölken MP, Boland MR, et al. ABC Index: quantifying experienced burden of COPD in a discrete choice experiment and predicting costs. BMJ Open. 2017;7(12):e017831. https://doi.org/10.1136/bmjopen-2017-017831.
Svedsater H, Roberts J, Patel C, Macey J, Hilton E, Bradshaw L. Life impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groups. Adv Ther. 2017;34:1466–81.
Disler RT, Green A, Luckett T, et al. Experience of advanced chronic obstructive pulmonary disease: metasynthesis of qualitative research. J Pain Symptom Manage. 2014;48(6):1182–99. https://doi.org/10.1016/j.jpainsymman.2014.03.009.
Kawata AK, Kleinman L, Harding G, Ramachandran S. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;7(4):413–26. https://doi.org/10.1007/s40271-014-0064-1.
Tervonen T, Hawken N, Martinez FJ, Heidenreich S, Gilbert I. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020;75(9):735–43. https://doi.org/10.1136/thoraxjnl-2019-213974.
Hawken N, Torvinen S, Neine ME, et al. Patient preferences for dry powder inhaler attributes in asthma and chronic obstructive pulmonary disease in France: a discrete choice experiment. BMC Pulm Med. 2017;17(1):99. https://doi.org/10.1186/s12890-017-0439-x.
Bridges JF, Kinter ET, Kidane L, Heinzen RR, McCormick C. Things are looking up since we started listening to patients: trends in the application of conjoint analysis in health 1982–2007. Patient. 2008;1(4):273–82. https://doi.org/10.2165/01312067-200801040-00009.
Slejko JF, Mattingly TJ, Mullins CD, Perfetto EM, dosReis S. Future of patients in healthcare evaluation: the patient-informed reference case. Value Health. 2019;22(5):545–8. https://doi.org/10.1016/J.JVAL.2019.02.003.
Mott DJ. Incorporating quantitative patient preference data into healthcare decision making processes: is HTA falling behind? Patient. 2018;11(3):249–52. https://doi.org/10.1007/s40271-018-0305-9.
Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–69. https://doi.org/10.1183/09031936.00099306.
Jones PW, Agusti AGN. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(4):822–32. https://doi.org/10.1183/09031936.06.00145104.
US Food and Drug Administration. Guidance for industry patient-reported outcome measures use in medical product development to support labeling claims. 2009. https://www.fda.gov/media/77832/download. Accessed 22 Jan 2021.
Garrison LP, Neumann PJ, Willke RJ, et al. A health economics approach to US value assessment frameworks: summary and recommendations of the ISPOR Special Task Force Report [7]. Value Health. 2018;21(2):161–5. https://doi.org/10.1016/j.jval.2017.12.009.
Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2(1):55–64.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–7. https://doi.org/10.2165/00019053-200826080-00004.
Sullivan J, Pravosud V, Mannino DM, Siegel K, Choate R, Sullivan T. National and state estimates of COPD morbidity and mortality: United States, 2014–2015. Chronic Obstr Pulm Dis. 2018;5(4):324–33. https://doi.org/10.15326/jcopdf.5.4.2018.0157.
Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease: United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205–11. https://doi.org/10.15585/mmwr.mm6707a1.
Acknowledgements
The authors thank Dr. Robert Wise of Johns Hopkins Bayview Medical Center for his efforts in the study advertisement, which led to patient recruitment necessary to complete the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by a grant from the Pharmaceutical Researchers and Manufacturers of America (PhRMA) Foundation Center of Excellence in Value Assessment Award. The views expressed in this manuscript were not contingent upon approval from or influenced by the PhRMA Foundation. Additional funding by the PhRMA supported this study.
Conflict of Interest
Julia F. Slejko reports grants from GSK Pharmaceuticals, Novartis Pharmaceuticals, and Takeda Pharmaceuticals and a teaching honorarium from Pfizer, all outside the submitted work. Susan dosReis reports grants from the National Institute of Mental Health, the Patient Centered Outcomes Research Institute, the US Food and Drug Administration, PhRMA Foundation, and GlaxoSmithKline. Yoon Duk Hong, Jamie L. Sullivan, and Robert M. Reed have no conflicts of interest that are directly relevant to the content of this article.
Availability of Data and Material
The ESM provides the instrument used to collect all the data summarized at each phase of this formative work. This enables transparency and replication of this study in other populations. There are no software codes or analytical models related to this work.
Author Contributions
JFS and SDR obtained funding for this work, conceptualized the design and data collection, led the data analysis, contributed to the interpretation of the findings, and drafted and edited all aspects of the manuscript. JLS and RMR contributed to participant recruitment, data interpretation and analysis, and reviewed and edited all versions of the manuscript. YDH assisted with the data collection and the summation of the findings and reviewed and edited all versions of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Slejko, J.F., Hong, Y.D., Sullivan, J.L. et al. Prioritization and Refinement of Patient-Informed Value Elements as Attributes for Chronic Obstructive Pulmonary Disease Treatment Preferences. Patient 14, 569–579 (2021). https://doi.org/10.1007/s40271-021-00495-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-021-00495-2