Abstract
Background
Growing international interest in mechanisms to capture and measure experiences and outcomes of health interventions from the patient perspective has led to the development of patient-reported measures (PRMs) across many areas of medicine. Although PRMs are now well utilised in some settings, the rapidly expanding area of direct-acting antiviral (DAA) treatments for hepatitis C has received remarkably little attention. In addition, questions are also being raised about the extent to which patients have been involved in the development of PRMs, which are primarily designed to reflect the patient perspective. In this context, the aim of this paper was to explore the possibility of developing a new PRM for use in hepatitis C DAA therapy that would also be acceptable to the patient group, in this case people who inject drugs (PWID).
Method
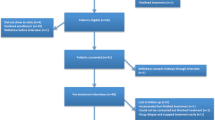
The study was based on a participatory design that included a peer researcher and foundational qualitative research including semi-structured interviews with 24 PWID with hepatitis C to inform the development of the PRMs. Stage 2 included four focus groups of six PWID with hepatitis C, who were asked to complete the draft measures and provide feedback.
Results
Participants responded positively to the draft PRMs. The results indicate that participants’ concerns during DAA treatment are often not sufficiently attended to in clinical settings. In the light of this finding, participants reported that PRMs have a positive role to play in the negotiation of patients’ care during DAA treatment.
Conclusions
The findings show that utilising a participatory approach to the development of PRMs for DAA HCV treatment with PWID not only provides a way to measure experiences and outcomes of treatment from the patient perspective, but also provides a means for highly marginalised patient groups to have a say in and negotiate their care in ways that might not otherwise be possible.
Similar content being viewed by others
References
Greenhalgh J, Dalkin S, Gooding K, Gibbons E, Wright J, Meads D, Health Services and Delivery Research, et al. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Serv Deliv Res. 2017;5(2):1–280.
Basch E. Patient-reported outcomes—harnessing patients’ voices to improve clinical care. N Engl J Med. 2017;376(2):105–8.
Williams K, Sansoni J, Morris D, Grootemaat P, Thompson C. Patient-reported outcome measures: literature review. Sydney: ACSQHC; 2016.
Basch E. New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med. 2014;65:307–17.
Black N. Patient reported outcome measures could help transform healthcare. BMJ Brit Med J. 2013;346:f167.
Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf. 2014;23(6):508–18.
Rose D, Thornicroft G, Slade M. Who decides what evidence is? Developing a multiple perspectives paradigm in mental health. Acta Psychiatr Scand. 2006;113:109–14.
Staniszewska S, Haywood KL, Brett J, Tutton L. Patient and public involvement in patient-reported outcome measures. Patient Patient Centered Outcomes Res. 2012;5(2):79–87.
Greenhalgh J, Long AF, Flynn R. The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Soc Sci Med. 2005;60(4):833–43.
Rose D, Evans J, Sweeney A, Wykes T. A model for developing outcome measures from the perspectives of mental health service users. Int Rev Psychiatry. 2011;23(1):41–6.
Brédart A, Marrel A, Abetz-Webb L, Lasch K, Acquadro C. Interviewing to develop Patient-Reported Outcome (PRO) measures for clinical research: eliciting patients’ experience. Health Qual Life Outcomes. 2014;12(1):15.
Rose D. Patient and public involvement in health research: ethical imperative and/or radical challenge? J Health Psychol. 2014;19(1):149–58.
Neale J, Strang J. Philosophical ruminations on measurement: methodological orientations of patient reported outcome measures (PROMS). J Ment Health. 2015;24(3):123–5.
Dawson J, Doll H, Fitzpatrick R, Jenkinson C, Carr AJ. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186.
Falade-Nwulia O, Suarez-Cuervo C, Nelson D, Fried M, Segal J, Sulkowski M. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–48.
Pawlotsky JM, Hepatitis C. Drugs: is next generation the last generation? Gastroenterology. 2016;151(4):587–90.
Feld JJ, Foster GR. Second generation direct-acting antivirals—do we expect major improvements? J Hepatol. 2016;65(1 Suppl):S130–42.
World Health Organisation. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. Geneva: World Health Organisation; 2016.
Hajarizadeh B, Grebely J, Dore G. Epidemiology and natural history of hepatitis C virus infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–62.
Dawson J. Measuring health status. In: Neale J, editor. Research methods for health and social care. London: Palgrave; 2009.
Brener L, Resnick I, Ellard J, Treloar C, Bryant J. Exploring the role of consumer participation in drug treatment. Drug Alcohol Depend. 2009;105:172–5.
Lloyd C. The stigmatization of problem drug users: a narrative literature review. Drugs Educ Prev Policy. 2013;20(2):85–95.
Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88:188–96.
Hatzenbuehler M, Phelan J, Link B. Stigma as a fundamental cause of population health inequalities. Am J Public Health. 2013;103:813–21.
Harris M. Managing expense and expectation in a treatment revolution: problematizing prioritisation through an exploration of hepatitis C treatment ‘benefit’. Int J Drug Policy. 2017;47:161–8.
Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity–establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1–eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Younossi ZM, Afendy A, Stepanova M, Racila A, Nader F, Gomel R, et al. Development and validation of a primary sclerosing cholangitis-specific patient-reported outcomes instrument: the PSC PRO. Hepatology. 2018;68(1):155–65.
Guidance for industry. patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? forthcoming.
Madden A, Hopwood M, Neale J, Treloar C. Beyond cure: patient reported outcomes of hepatitis C treatment among people who inject drugs. forthcoming.
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12(8):1075–83.
Treloar C, Newland J, Harris M, Deacon R, Maher L. A diagnosis of hepatitis C: insights from a study on patients’ experiences. Aust Fam Physician. 2010;39(8):589–92.
Snow K, Scott N, Clothier HJ, MacLachlan JH, Cowie B. Limited provision of diagnostic services to Victorians living with hepatitis C antibodies, 2001–2012: a multi-level modelling analysis. Aust N Z J Public Health. 2016. https://doi.org/10.1111/1753-6405.12560.
Kyte DG, Calvert M, van der Wees PJ, ten Hove R, Tolan S, Hill JC. An introduction to patient-reported outcome measures (PROMs) in physiotherapy. Physiotherapy. 2015;101(2):119–25.
Treloar C, Rance J, Yates K, Mao L. Trust and people who inject drugs: the perspectives of clients and staff of Needle Syringe Programs. Int J Drug Policy. 2016;27:138–45.
Merrill J, Rhodes L, Deyo RA, Marlatt G, Bradley K. Mututal mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17:327–33.
Mechanic D, Meyer S. Concepts of trust among patients with serious illness. Soc Sci Med. 2000;51:657–68.
Treloar C, Rhodes T. The lived experience of hepatitis C and its treatment among injecting drug users: qualitative synthesis. Qual Health Res. 2009;19(9):1321–34.
Grebely J, Applegate TL, Cunningham P, Feld JJ. Hepatitis C point-of-care diagnostics: in search of a single visit diagnosis. Expert Rev Mol Diagn. 2017;17(12):1109–15.
Madden A, Lea T, Bath N, Winstock AR. Satisfaction guaranteed? What clients on methadone and buprenorphine think about their treatment. Drug Alcohol Rev. 2008;27(6):671–8.
Acknowledgements
We wish to thank the participants in this study. We are grateful for the support of Harm Reduction Victoria and Hepatitis Victoria. Joanne Neale is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Annie Madden is currently a PhD candidate at the Centre for Social Research in Health and is supported through an Australian Government Research Training Program Scholarship. The Centre for Social Research in Health is supported by a grant from the Australian Government Department of Health. This project was supported by a seed grant from the PLuS Alliance.
Author information
Authors and Affiliations
Contributions
CT and JN designed the study. All authors contributed to the data collection tools. AM collected data. MH supervised data collection. AM conducted initial analysis supervised by MH. AM, MH, JN and CT contributed to the draft manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
This study was funded by the PLuS Alliance and supported by a grant from the Australian Government Department of Health. Annie Madden, Max Hopwood, Joanne Neale and Carla Treloar have no financial interests to declare. Annie Madden has experience of injecting drug use and of receiving treatment for hepatitis C with direct-acting antiviral therapies and has more than 20 years’ experience as an advocate for the rights of people who inject drugs with local and national peer-based organisations run by and for people who use/have used illicit drugs.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data availability statement
The datasets generated during the current study are not publicly available given the sensitive nature of the information collected but are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Madden, A., Hopwood, M., Neale, J. et al. Acceptability of Patient-Reported Outcome and Experience Measures for Hepatitis C Treatment Among People Who Use Drugs. Patient 12, 259–265 (2019). https://doi.org/10.1007/s40271-018-0332-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-018-0332-6