Abstract
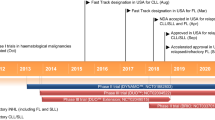
Umbralisib (UKONIQ™) is an oral, first-in-class dual phosphatidylinositol 3-kinase delta (PI3Kδ) and casein kinase 1 epsilon (CK1ε) inhibitor being developed by TG Therapeutics for the treatment of various haematological malignancies. In February 2021, umbralisib received its first approval in the USA for the treatment of adults with relapsed or refractory marginal zone lymphoma (MZL) who have received ≥ 1 prior anti-CD20-based regimen, and relapsed or refractory follicular lymphoma (FL) who have received ≥ 3 prior lines of systemic therapy. Clinical studies in various haematological malignancies, including chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma, are underway in multiple countries. This article summarizes the milestones in the development of umbralisib leading to this first approval.
Similar content being viewed by others
References
Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26.
Jabbour E, Ottmann OG, Deininger M, et al. Targeting the phosphoinositide 3-kinase pathway in hematologic malignancies. Haematologica. 2014;99(1):7–18.
US FDA. FDA grants accelerated approval to umbralisib for marginal zone lymphoma and follicular lymphoma [media release]. 5 Feb 2021. https://www.fda.gov/.
TG Therapeutics. UKONIQ™ (umbralisib): US prescribing information. 2021. https://www.tgtherapeutics.com/. Accessed 23 Feb 2021.
TG Therapeutics. TG Therapeutics and Rhizen Pharmaceuticals announce global agreement for development and commercialization of novel PI3K Delta selective inhibitor, TGR-1202 [media release]. 16 Aug 2012. https://ir.tgtherapeutics.com/.
TG Therapeutics. TG Therapeutics announces exercise of license option for its novel, next generation PI3K-Delta inhibitor, TGR-1202 [media release]. 23 Sep 2014. https://ir.tgtherapeutics.com/
Rhizen Pharmaceuticals. Rhizen Pharmaceuticals announces out-licensing agreement for TGR-1202, a novel next generation PI3K-delta inhibitor [media release]. 23 Sep 2014. http://www.rhizen.com.
Deng C, Lipstein M, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3K Delta and CK1 epsilon in hematological malignancies [abstract]. Blood. 2016;128(22):291.
Burris HA 3rd, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19(4):486–96.
Maharaj K, Powers JJ, Achille A, et al. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020;4(13):3072–84.
Locatelli SL, Careddu G, Inghirami G, et al. The novel PI3K-δ inhibitor TGR-1202 enhances brentuximab vedotin-induced Hodgkin lymphoma cell death via mitotic arrest. Leukemia. 2016;30(12):2402–5.
Pal I, Sardinha AG, Scotto L, et al. Umbralisib and carfilzomib potently inhibit cap dependent translation in lymphoma [abstract no. 483]. Hematol Oncol. 2019;37(Suppl 2):510–1.
Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kdelta/CK1epsilon inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.20.03433.
Mato AR, Ghosh N, Schuster SJ, et al. Phase 2 study of the safety and efficacy of umbralisib in patients with CLL who are intolerant to BTK or PI3Kδ inhibitor therapy. Blood. 2020. https://doi.org/10.1182/blood.2020007376.
Gribben JG, Jurczak W, Jacobs R, et al. Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naive (TN) and relapsed/ refractory (R/R) chronic lymphocytic leukemia (CLL): results from the phase 3 Unity-CLL study [abstract no. 543 plus oral presentation]. Blood. 2020;136(Suppl 1):37–9.
Davids MS, Kim HT, Nicotra A, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1–1b study. Lancet Haematol. 2019;6(1):e38–47.
Lunning M, Vose J, Nastoupil L, et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(21):1811–20.
Lunning M, Bierman P, Bociek R, et al. A phase IIa study evaluating the safety and tolerability of umbralisib and ibrutinib in patients with relapsed or refractory diffuse large B-cell lymphoma [abstract no. 494]. Hematol Oncol. 2019;37(Suppl 2):519.
Ramchandren R, Mulroney CM, Patel MR, et al. A phase I trial of TGR-1202, a next generation once-daily PI3Kdelta inhibitor, in combination with brentuximab vedotin, in patients with relapsed/refractory Hodgkins lymphoma [abstract plus poster]. Blood. 2016;128(22):4146.
Barr PM, Ma S, Zent CS, et al. A phase 1/2 study of umbralisib, ublituximab, and venetoclax (U2-Ven) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) [abstract no. 3137 plus oral presentation]. Blood. 2020;136(Suppl. 1):41–2.
Mato AR, Svoboda J, Luning Prak ET, et al. Phase I/II study of umbralisib (TGR-1202) in combination with ublituximab (TG-1101) and pembrolizumab in patients with rel/ref CLL and Richter’s transformation [abstract no. 073 plus oral presentation]. Hematol Oncol. 2019;37(Suppl 2):119–20.
Nastoupil LJ, Lunning MA, Vose JM, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 2019;6(2):e100–9.
TG Therapeutics. TG Therapeutics provides business update and reports fourth quarter and year-end 2020 financial results [media release]. 2 Mar 2021. https://ir.tgtherapeutics.com/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon and Susan Keam are contracted/salaried employees of Adis International Ltd/Springer Nature and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Dhillon, S., Keam, S.J. Umbralisib: First Approval. Drugs 81, 857–866 (2021). https://doi.org/10.1007/s40265-021-01504-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01504-2