Abstract
Intravenous durvalumab (Imfinzi™; AstraZeneca) is a fully human monoclonal antibody that blocks programmed cell death ligand-1 binding to its receptors (PD-1 and CD80), resulting in enhanced T-cell responses against cancer cells. The US FDA has granted durvalumab accelerated approval for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy. Durvalumab ± tremelimumab is under phase III clinical trials in urothelial carcinoma, non-small cell lung cancer, small cell lung cancer and head and neck squamous cell carcinoma. The drug is also being evaluated in phase I or II clinical trials in a wide range of solid tumours and haematological malignancies. This article summarizes the milestones in the development of durvalumab leading to this first approval for urothelial carcinoma.
Similar content being viewed by others
1 Introduction
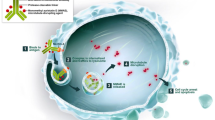
Durvalumab (Imfinzi™) is a fully human immunoglobulin G1k monoclonal antibody against programmed cell death ligand-1 (PD-L1) that is being developed by AstraZeneca for the treatment of cancer [1, 2]. PD-L1 binds to programmed cell death-1 (PD-1) and CD80 (B7-1) receptors, resulting in inhibition of T-cell function. A broad range of human tumours upregulate PD-L1, evading immune surveillance and antitumour T-cell responses. Thus, overexpression of PD-L1 is associated with poor prognosis. Durvalumab blocks PD-L1 binding to both PD-1 and CD80, resulting in enhanced recognition and killing of tumour cells by T-cells [1, 2].
Intravenous durvalumab received US FDA accelerated approval in May 2017 for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy [3]. The approval was based on the objective response rate (ORR) and duration of response seen in Study 1108 (Sect. 2.3.1) and continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials [3]. The recommended dosage of durvalumab is 10 mg/kg intravenous infusion over 60 min every 2 weeks until disease progression or unacceptable toxicity [3]. Withholding or discontinuing durvalumab is recommended to manage adverse events, such as pneumonitis, hepatitis, colitis or diarrhoea, hypothyroidism, adrenal insufficiency, hypophysitis/hypopituitarism, type 1 diabetes mellitus, nephritis, rash or dermatitis, infection, infusion-related reactions or other grade 3 or 4 adverse events [3]. The US FDA granted durvalumab a breakthrough therapy designation in PD-L1-positive urothelial bladder cancer in February 2016 [4] and a priority review status in bladder cancer in December 2016 [5].

Key milestones in the development of durvalumab. Est estimated completion date, NSCLC non-small cell lung cancer, HNSCC head and neck squamous cell carcinoma, SCLC small cell lung cancer, UC urothelial cancer
Phase III development of durvalumab monotherapy or in combination with tremelimumab is underway in urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC) and head and neck squamous cell carcinoma (HNSCC). Durvalumab is also being evaluated extensively in phase I or II clinical trials in a wide range of solid tumours and haematological malignancies.
1.1 Company Agreements
AstraZeneca or MedImmune (a subsidiary of AstraZeneca) have collaboration agreements with pharmaceutical companies to evaluate durvalumab in combination with the following drugs in early phase trials:
-
Mirati Therapeutics’ mocetinostat in NSCLC (agreement signed in August 2015) [6].
-
Peregrine’s bavituximab in solid tumours, including NSCLC (August and October 2015) [7].
-
Eli Lilly’s ramucirumab, galunisertib, LY2510924 or LY3022855 in solid tumours (August and October 2015) [8].
-
Celgene’s anticancer drugs in haematological malignancies (April 2015) [9].
-
Gilead’s idelalisib in haematological cancers/solid tumours, including diffuse large B-cell lymphoma and triple-negative breast cancer (first quarter of 2015) [10].
-
Innate Pharma’s monalizumab in cancer (April 2015) [11].
-
Pharmacyclics (a subsidiary of AbbVie) and Janssen Biotech’s ibrutinib in solid tumours and haematological malignancies (November 2014) [12].
-
Immunocore’s IMC gp100 in melanoma (April 2015) [13].
-
Advaxis’ axalimogene filolisbac in human papillomavirus (HPV)-associated cervical cancer and HNSCC (July 2014) [14].
-
Kyowa Hakko Kirin’s mogamulizumab in solid tumours (July 2014) [15].
2 Scientific Summary
2.1 Pharmacodynamics
Durvalumab binds to PD-L1 with high affinity and specificity, blocking its interaction with PD-1 and CD80 receptors [2]. Durvalumab does not bind to PD-L2 [2]. It is specifically engineered to disable cytotoxic effector functions, such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity against cells expressing PD-L1 [1, 2]. Durvalumab inhibited the activity of PD-L1 in a concentration-dependent manner in an anti-CD3-based T-cell activation assay and a mixed lymphocyte reaction assay [2]. Durvalumab inhibited tumour growth in mouse xenograft models of human melanoma (A375) and pancreatic (HPAC) tumour cell lines, via a T-cell-mediated mechanism [2].
Data from the Study 1108 (Sect. 2.3.1) suggest that patients with NSCLC who had detectable levels of interferon γ mRNA and PD-L1 protein expression in ≥25% of tumour cells may have longer survival with durvalumab treatment; durvalumab significantly (p < 0.016) induced interferon γ gene expression in tumour microenvironment [16].
In a phase I study in patients with advanced NSCLC, durvalumab plus tremelimumab combination therapy was associated with higher peripheral T-cell activation and proliferation, compared with durvalumab monotherapy [17]. Durvalumab plus MEDI0680 (a monoclonal antibody that blocks interaction of PD-L1 and PD-L2 with PD-1 receptors) treatment was associated with increased pharmacodynamic activity against the PD-1/PD-L1 pathway in patients with advanced malignancies in a phase I study [18]. Durvalumab did not appear to antagonize the gefitinib’s mechanism of action [epidermal growth factor receptor (EGFR) inhibition] in patients with NSCLC in a phase I study [19].
2.2 Pharmacokinetics
Exposure to durvalumab increased dose-proportionally at ≥3 mg/kg in cancer patients, with steady state levels observed at ≈16 weeks [3]. The geometric mean steady state volume of distribution was 5.6 L [3]. As with therapeutic monoclonal antibodies in general, durvalumab undergoes target-mediated clearance [20]. Durvalumab clearance decreases over time, but is not considered clinically relevant; the geometric mean terminal half-life of durvalumab is ≈17 days [3].
The pharmacokinetics of durvalumab are not affected to a clinical significant extent by age (19–96 years), bodyweight (34–149 kg), sex, race, tumour type, Eastern Cooperative Oncology Group performance status, levels of albumin, lactate dehydrogenase, creatinine or soluble PD-L1, mild or moderate renal impairment [creatinine clearance (CLCR) 60–89 and 30–59 mL/min, respectively) or mild hepatic impairment [bilirubin ≤ upper limit of normal (ULN) and AST >ULN or bilirubin >1.0 to 1.5 times ULN and any AST] [3]. The effect of severe renal impairment (CLCR 15–29 mL/min), or moderate or severe hepatic impairment (bilirubin >1.5 to 3.0 and >3.0 times ULN, respectively, and any AST) on the pharmacokinetics of durvalumab is unknown [3].
There was no clinically relevant pharmacokinetic interaction between durvalumab and tremelimumab [17] or durvalumab and gefitinib [21] in patients with NSCLC.
2.3 Therapeutic Trials
2.3.1 Urothelial Carcinoma
In a multicentre, multicohort, open-label, phase I/II trial (Study 1108; NCT01693562), a cohort of patients with urothelial carcinoma (n = 182) treated with durvalumab had an ORR of 17.6% (95% CI 12.3−23.9) in all patients, 27.4% (18.7−37.5) in the PD-L1-high subgroup (n = 95) and 4.1% (0.9−11.5) in the PD-L1-low/negative subgroup (n = 73) (abstract presentation [22]). Among 32 responders, 15 (46.9%) had ongoing responses of ≥6 months. Study 1108 included patients with inoperable or metastatic solid tumours. The urothelial carcinoma cohort had progressed while receiving or after a platinum-based therapy, including those who progressed within 12 months of receiving therapy in a neo-adjuvant or adjuvant setting. Patients received durvalumab 10 mg/kg every 2 weeks for up to 12 months or until unacceptable toxicity or disease progression. The ORR was assessed using the RECIST v1.1 criteria by a blinded independent central review [22].
2.3.2 Non-small Cell Lung Cancer
In the randomized, double-blind, placebo-controlled, phase III PACIFIC trial (NCT02125461), a planned interim analysis showed that durvalumab sequential monotherapy significantly prolonged progression-free survival in patients with unresectable stage III NSCLC who had not progressed following platinum-based concurrent chemoradiation therapy (media release presentation [23]).
In Study 1108, durvalumab monotherapy was associated with improved ORR and overall survival in patients with PD-L1-positive NSCLC (abstract presentation [24]). After a median six doses, the confirmed ORR was 25% in the PD-L1-positive (n = 154) and 6% in the PD-L1-negative (n = 115) subgroups, with a similar trend seen in the first-line (29 and 11%), second-line (26 and 4%) and ≥3rd line (22 and 6%) setting. The median overall survival in the PD-L1-positive and PD-L1-negative subgroups was: second-line 17.8 and 8.2 months; ≥3rd line 13.0 and 7.1 months. The 12-month overall survival rate was: second-line 55.8 and 38.8%; ≥3rd line 51.1 and 36.8% [24]. Durvalumab treatment showed early clinical benefit in treatment-naive patients with advanced NSCLC of squamous or nonsquamous histology [25].
In a phase Ib dose-escalation study (NCT02000947), a combination of durvalumab plus tremelimumab showed early clinical activity in immunotherapy-naive patients with advanced NSCLC, regardless of PD-L1 status (interim data) [17]. Patients received durvalumab 3–20 mg/kg every 4 weeks or 10 mg/kg every 2 weeks and tremelimumab 1–10 mg/kg every 4 weeks for six doses then every 12 weeks for 12 months. In all evaluable patients with ≥24 weeks follow-up, the ORR was 29% across all dosage cohorts, 33% in the PD-L1-positive subgroup and 30% in the PD-L1-negative subgroup (n = 63, 18 and 37, respectively). The corresponding disease control rate at ≥24 weeks was 41, 39 and 46%. Durvalumab 20 mg/kg plus tremelimumab 1 mg/kg every 4 weeks was selected for phase III studies [17].
In a phase I study (NCT02088112), a combination of durvalumab and gefitinib showed early clinical activity in tyrosine kinase inhibitor-naive patients with EGFR mutant advanced NSCLC [21].
2.3.3 Head and Neck Squamous Cell Carcinoma
In Study 1108, durvalumab monotherapy was associated with durable responses in a cohort of patients with recurrent or metastatic HNSCC previously treated with a median of three systemic treatments (n = 62) (abstract presentation [26]). In this cohort, retreatment was permitted upon progression after 12 months. After a median follow-up of 25 months, there were seven responders, with six patients showing response duration of ≥12 months. The 12-month overall survival was 42%, with no clear difference by PD-L1 status [26].
2.3.4 Other Cancers
Durvalumab alone or in combination with other drugs showed evidence of clinical activity in the following trials (abstract presentations):
-
Durvalumab monotherapy in an open-label phase II trial (NCT02336165) in bevacizumab-naive patients with recurrent glioblastoma (estimated 6-month progression-free survival 20.0%; ORR 13.3%; stable disease rate 40.0%; n = 30) [27].
-
Durvalumab monotherapy in a phase I trial (NCT01938612) in Japanese patients with advanced solid tumours [28].
-
Durvalumab plus olaparib in a phase II trial (NCT02484404) in previously treated patients with metastatic castration-resistant prostate cancer (6-month progression-free survival 86.7%, 9-month progression-free survival 57.8%; n = 19) [29] and in a phase I (NCT02484404) trial in patients with ovarian cancer or triple-negative breast cancer without germline BRCA mutation [30].
-
Durvalumab plus axalimogene filolisbac in a phase I/II trial (NCT02291055) in previously treated patients with recurrent/metastatic HPV-associated cervical cancer [31].
-
Durvalumab plus darafenib plus trametinib in a phase I/II trial (NCT02027961) in patients with BRAF mutant or wild type metastatic or unresectable melanoma [32].
-
Durvalumab plus tremelimumab in a phase I trial (NCT02141347) in Japanese patients with advanced solid tumours [33].
-
Durvalumab plus MEDI0680 in a phase I trial (NCT02118337) in patients with advanced malignancies [18].
-
Durvalumab plus AZD9150 (antisense oligonucleotide against STAT3) or AZD5069 (CXCR2 antagonist) in a phase Ib/II trial (NCT02499328) in patients with advanced malignancies [34].
Key clinical trials of durvalumab (sponsored by AstraZeneca/MedImmune unless stated otherwise)
Drug(s) | Indication | Phase | Status | Identifier |
|---|---|---|---|---|
Durvalumab ± tremelimumab, SoC (1st line) | Stage IV urothelial carcinoma | III | Recruiting | NCT02516241; DANUBE |
Durvalumab, SoC (1st line) | Advanced NSCLC | III | Recruiting | NCT03003962; PEARL |
Durvalumab ± tremelimumab, SoC (1st line) | Advanced/metastatic NSCLC | III | Ongoing | NCT02453282; MYSTIC |
Durvalumab + tremelimumab, SoC (1st line) | Advanced/metastatic NSCLC | III | Recruiting | NCT02542293; NEPTUNE |
Durvalumab ± tremelimumab, SoC (3rd line) | Advanced/metastatic NSCLC | III | Ongoing | NCT02352948; ARCTIC |
Durvalumab + concurrent chemoradiation | Stage III unresectable NSCLC | III | Ongoing | NCT02125461; PACIFIC |
Durvalumab, placebo (adjuvant therapy) | Completely resected NSCLC | III | Recruiting | NCT02273375; ADJUVANTa |
Durvalumab, multiple comparators (biomarker-targeted 2nd line) | Stage IV squamous NSCLC | II/III | Recruiting | NCT02154490; Lung Master Protocola |
Durvalumab (3rd line) | Advanced/metastatic NSCLC | II | Ongoing | NCT02087423; ATLANTIC |
Durvalumab ± tremelimumab + chemotherapy | Advanced SCLC | III | Recruiting | NCT03043872; CASPIAN |
Durvalumab + tremelimumab, AZD1775 + carboplatin | Extensive stage SCLC | II | Recruiting | NCT02937818; BALTIC |
Durvalumab ± tremelimumab, SoC (1st line) | Recurrent/metastatic HNSCC | III | Ongoing | NCT02551159; KESTREL |
Durvalumab ± tremelimumab, SoC (2nd line) | Recurrent/metastatic HNSCC | III | Recruiting | NCT02369874; EAGLE |
Durvalumab | Recurrent/metastatic HNSCC | II | Ongoing | NCT02207530; HAWK |
Durvalumab, tremelimumab, durvalumab + tremelimumab | Recurrent/metastatic HNSCC | II | Ongoing | NCT02319044; CONDOR |
Durvalumab + tremelimumab, durvalumab, tremelimumab | Unresectable hepatocellular carcinoma | II | Recruiting | NCT02519348 |
Durvalumab + tremelimumab, durvalumab | Advanced solid tumours | III | Recruiting | NCT03084471; STRONG |
Durvalumab | HIV-1 plus solid tumours | II | Recruiting | NCT03094286 |
Durvalumab, tremelimumab, durvalumab + tremelimumab | Advanced solid tumours | II | Ongoing | NCT02527434 |
Durvalumab (≥2nd line) | Advanced solid tumours | I/II | Ongoing | NCT01693562; Study 1108 |
2.4 Adverse Events
Durvalumab had a manageable tolerability profile in patients with urothelial carcinoma in Study 1108 (n = 182; median duration of durvalumab exposure 10.2 weeks) [3]. Durvalumab treatment was delayed or interrupted in 31% of patients, most commonly because of liver injury (4.9%), urinary tract infection (3.3%), acute kidney injury (3.3%) and musculoskeletal pain (2.7%). Serious adverse reactions occurred in 46% of patients and the most common (incidence >2%) were acute kidney injury (4.9%), urinary tract infection (4.4%), musculoskeletal pain (4.4%), liver injury (3.3%), general physical health deterioration (3.3%), sepsis (2.7%), abdominal pain (2.7%) and pyrexia/tumour associated fever (2.7%) [3].
In Study 1108, adverse reactions of any grade and grade 3 or 4 were reported in 96 and 43% of patients respectively [3]. The most common adverse events (incidence ≥15%) of any grade were fatigue (39%), musculoskeletal pain (24%), constipation (21%), decreased appetite (19%), nausea (16%), peripheral oedema (15%) and urinary tract infection (15%). The most common (incidence ≥3%) grade 3 or 4 adverse reactions were fatigue (6%), urinary tract infection (4%), musculoskeletal pain (4%) and abdominal pain (3%), as well as dehydration and general physical health deterioration (incidence not reported). Grade 5 adverse events occurred in 4.4% of durvalumab recipients, including cardiorespiratory arrest, general physical health deterioration, sepsis, ileus, pneumonitis or immune-mediated hepatitis. The most common (incidence ≥4%) grade 3 or 4 laboratory abnormalities which worsened from baseline were hyponatremia (12%), lymphopenia (11%), anaemia (8%), increased alkaline phosphatase (4%) and hypermagnesemia (4%). Immune-mediated adverse reactions requiring systemic corticosteroids or hormone replacement therapy were reported in 8.2% of durvalumab recipients [3].
In Study 1108, palliative concurrent radiotherapy with durvalumab was generally well tolerated in a subgroup of patients (n = 10) with solid tumours, including urothelial bladder cancer [35].
Durvalumab plus tremelimumab had a manageable tolerability profile in patients with NSCLC in the NCT02000947 trial discussed in Sect. 2.3.2 (n = 102; median follow-up 18.8 weeks) [17]. The most common treatment-related grade 3 or 4 adverse events were diarrhoea (11%), colitis (9%) and increased lipase (8%). Treatment-related serious adverse events occurred in 36% of patients, with 28% of patients discontinuing treatment attributable to treatment-related adverse events. There were three treatment-related deaths because of complications arising from myasthenia gravis, pericardial effusion and neuromuscular disorder [17].
As with all therapeutic proteins, durvalumab has the potential for immunogenicity [3]. Among evaluable 1124 patients treated with durvalumab 10 mg/kg every 2 weeks, 3.3% of patients had treatment-emergent anti-durvalumab antibodies. The clinical significance these antibodies is currently unknown [3].
2.5 Complementary Diagnostic
In May 2017, the US FDA approved Roche’s VENTANA PD-L1 (SP263) assay as a complementary diagnostic for the qualitative detection of PD-L1 expression in patients with locally advanced or metastatic urothelial carcinoma who are being considered for treatment with durvalumab [36]. The assay is available in the USA for use on the BenchMark ULTRA staining instrument and may be useful in determining the likelihood of responding to durvalumab, but not required for use of durvalumab [36].
2.6 Ongoing Clinical Trials
Durvalumab alone or in combination with other drugs is being evaluated in more than 150 clinical trials [sponsored by AstraZeneca/MedImmune, partnered companies (Sect. 1.1) or investigators] in a wide range of solid tumours and haematological malignancies. A selected list of clinical trials based on AstraZeneca’s durvalumab pipeline reported in the first quarter of 2017 [37] is presented here.
The DANUBE trial in urothelial carcinoma is ongoing. Furthermore, a biomarker-directed phase Ib trial (BISCAY; NCT02546661) is evaluating durvalumab alone or in combination with AZD4547 (fibroblast growth factor receptor tyrosine kinase 1, 2 and 3 inhibitor), AZD1775 (WEE1 tyrosine kinase inhibitor), olaparib or vistusertib in patients with muscle invasive bladder cancer.
Phase III trials evaluating durvalumab as monotherapy (PEARL, MYSTIC, ARCTIC, PACIFIC) or in combination with tremelimumab (MYSTIC, NEPTUNE, ARCTIC) in the first-line (PEARL, MYSTIC, NEPTUNE), second-line (PACIFIC) or third-line (ARCTIC) settings in NSCLC are ongoing. The ADJUVANT, Lung Master Protocol and ATLANTIC trials, and the clinical trials discussed in Sect. 2.3.2 (Study 1108, NCT02000947, NCT02088112) are also ongoing. In addition, AstraZeneca has initiated a phase III trial (POSEIDON; NCT03164616) to evaluate the addition of durvalumab plus tremelimumab to the standard-of-care in patients with metastatic NSCLC. The CASPIAN and BALTIC trials in patients with SCLC are ongoing.
The KESTREL, EAGLE, HAWK and CONDOR trials in HNSCC are ongoing. The NCT02291055 and NCT02499328 trials (Sect. 2.3.4) in expanded cohorts of HNSCC are ongoing. In addition, a phase I trial (NCT02262741) is evaluating durvalumab plus tremelimumab in patients with HNSCC.
Durvalumab is also being evaluated in the following trials: a phase Ib/II trial (NCT02549651) of durvalumab alone and in combination with tremelimumab or AZD9150 in diffuse large B-cell lymphoma; a phase Ib/II trial (Study 21; NCT02340975) of durvalumab alone and in combination with tremelimumab in gastric adenocarcinoma; the NCT02027961 trial in melanoma (Sect. 2.3.4); and, phase I trials of durvalumab in combination with MEDI0680 (NCT02118337; Sect. 2.3.4), MEDI0526 (NCT02705482), monalizumab (NCT02671435), selumetinib (NCT02586987), AZD1775 (NCT02617277), MEDI9447 (NCT02503774), tremelimumab in Japanese patients (NCT01938612) and tremelimumab plus first-line chemotherapy (NCT02658214) in advanced solid tumours.
3 Current Status
Durvalumab received its first global approval on 1 May 2017 in the USA for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy.
Change history
10 October 2017
A correction to this article has been published.
References
Ibrahim R, Stewart R, Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol. 2015;42(3):474–83.
Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–62.
Imfinzi™ (durvalumab): US prescribing information. 2017. https://www.fda.gov. Accessed 24 May 2017.
AstraZeneca. Durvalumab granted Breakthrough Therapy designation by US FDA for treatment of patients with PD-L1 positive urothelial bladder cancer [media release]. 17 Feb 2016. http://www.astrazeneca.com.
AstraZeneca. US FDA accepts first biologics license application for AstraZeneca’s durvalumab in bladder cancer [media release]. 9 Dec 2016. http://www.astrazeneca.com.
AstraZeneca. MedImmune and Mirati Therapeutics partner on immuno-oncology combination in lung cancer [media release]. 5 Aug 2015. www.astrazeneca.com.
Peregrine Pharmaceuticals. AstraZeneca and Peregrine Pharmaceuticals expand ongoing immuno-oncology collaboration to include phase II lung cancer combination clinical trial [media release]. 15 Oct 2015. http://www.peregrineinc.com.
Eli Lilly. Lilly and AstraZeneca expand immuno-oncology research collaboration with new combinations [media release]. 22 Oct. 2015. http://www.lillyoncology.com.
Celgene International SARL. Celgene corporation enters into strategic immuno-oncology collaboration with Astrazeneca to develop PD-L1 inhibitor program for patients with serious blood cancers [media release]. 24 April 2015. http://www.celgene.com.
AstraZeneca. AstraZeneca PLC first quarter results 2015 [media release]. 24 Apr 2015. http://www.astrazeneca.com.
Innate Immune. Innate Pharma and AstraZeneca announce global co-development and commercialisation agreement for IPH2201 in immuno-oncology [media release]. 24 April 2015. http://www.innate-pharma.com.
AstraZeneca, Pharmacyclics, Janssen Research Development. AstraZeneca, Pharmacyclics and Janssen partner on immuno-oncology combination trials with Imbruvica® for hematologic cancers [media release]. 4 Nov. 2014. http://www.astrazeneca.com.
AstraZeneca. Medimmune and Immunocore announce new collaboration to conduct immuno- oncology combination trials in melanoma [media release]. 16 April 2015. http://www.astrazeneca.com.
Advaxis. Advaxis and MedImmune partner on immuno-oncology combination clinical trial [media release]. 22 July 2014. http://www.advaxis.com.
Kyowa Hakko Kirin. Kyowa Hakko Kirin and AstraZeneca partner on immuno-oncology clinical study [media release]. 30 July2014. http://www.kyowa-kirin.com.
Higgs BW, Morehouse C, Streicher K, et al. Relationship of baseline tumoral IFNγ mRNA and PD-L1 protein expression to overall survival in durvalumab-treated NSCLC patients [abstract no. 3036]. J Clin Oncol. 2016;34(Suppl):3036.
Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308.
Hamid O, Chow LQ, Sanborn RE, et al. Combination of MEDI0680, an anti-PD-1 antibody, with durvalumab, an anti-PD-L1 antibody: a phase 1, open-label study in advanced malignancies [abstract no. 1050PD]. Ann Oncol. 2016;27(Suppl 6). doi:10.1093/annonc/mdw378.05.
Yeh T, Jacobs V, Angell H, et al. Inhibition of pEGFR in paired tumour biopsies from TKI treatment-naive EGFR mutant NSCLC patients treated with gefitinib (EGFR inhibitor) or gefitinib in combination with durvalumab (anti-PD-L1) [abstract no. 60PD]. J Thorac Oncol. 2016;11(Suppl 4):S80–1.
Song X, Pak M, Chavez C, et al. Population pharmacokinetics of MEDI4736, a fully human antiprogrammed death ligand 1 (PD-L1) monoclonal antibody, in patients with advanced solid tumors [abstract no. 203]. Eur J Cancer. 2015;51(Suppl 3):S28.
Gibbons DL, Chow LQ, Kim DW, et al. Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC [abstract no. 57O]. J Thorac Oncol. 2016;11(Suppl 4):S79.
Hahn NM, Powles T, Massard C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma (UC) [abstract no. 4525 plus poster]. J Clin Oncol. 2017;35(Suppl):286.
AstraZeneca. Imfinzi™ (durvalumab) significantly reduces the risk of disease worsening or death in the phase III PACIFIC trial for stage III unresectable lung cancer [media release]. 15 May 2017. https://www.astrazeneca-us.com.
Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC) [abstract no. 1216PD]. Ann Oncol. 2016;27(Suppl 6). doi:10.1093/annonc/mdw383.16.
Antonia SJ, Kim SW, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-LI antibody, in treatment-naive patients with advanced non-small-cell lung cancer [abstract no. 9029]. J Clin Oncol. 2016;34(Suppl):8032.
Segal NH, Ou SHI, Balmanoukian AS, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort [abstract no. 949O]. Ann Oncol. 2016;27(Suppl 6). doi:10.1093/annonc/mdw376
Reardon DA, Kaley TJ, Dietrich J, et al. Phase 2 study to evaluate safety and efficacy of MEDI4736 (durvalumab [DUR]) in glioblastoma (GBM) patients: an update [abstract no. 2042 plus poster]. J Clin Oncol. 2017;35(Suppl):TPS2080.
Iguchi H, Nogami N, Kozuki T, et al. Phase I study to evaluate the safety and tolerability of MEDI4736, an anti-programmed cell death ligand1 (PD-L1) antibody, in Japanese patients with advanced solid tumors [abstract no. 3039]. J Clin Oncol. 2015;33(Suppl):3039.
Karzai F, Madan RA, Owens H, et al. Combination of PDL-1 and PARP inhibition in an unselected population with metastatic castrate-resistant prostate cancer (mCRPC) [abstract no. 5026 plus poster]. J Clin Oncol. 2017;35(Suppl):5026.
Lee JM, Santos Zimmer AD, Lipkowitz S, et al. Phase I study of the PD-L1 inhibitor, durvalumab (MEDI4736; D) in combination with a PARP inhibitor, olaparib (O) or a VEGFR inhibitor, cediranib (C) in women’s cancers (NCT02484404) [abstract no. 3015]. J Clin Oncol. 2016;34(Suppl):3015.
Slomovitz BM, Moore KM, Youssoufian H, et al. A phase I/II study of durvalumab alone or in combination with AXAL in recurrent/persistent or metastatic cervical or human papillomavirus (HPV) + squamous cell cancer of the head and neck (SCCHN): preliminary phase I results [abstract no. P241]. J Immunother Cancer. 2016;4(Suppl 1):134.
Ribas A, Butler M, Lutzky J, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma [abstract no. 3003]. J Clin Oncol. 2015;33(Suppl):3003.
Takahashi Y, Fujikawa K, Sagawa T, et al. A phase 1 study to assess the safety and tolerability of tremelimumab alone and in combination with MEDI4736 in Japanese patients with advanced solid malignancies [abstract no. 512]. Eur J Cancer. 2015;51(Suppl 3):S107.
Hong D, Falchook G, Cook CE, et al. A phase 1b study (SCORES) assessing safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of durvalumab combined with AZD9150 or AZD5069 in patients with advanced solid malignancies and SCCHN [abstract no. 1049PD]. Ann Oncol. 2016;27(Suppl 6). doi:10.1093/annonc/mdw378.04.
Levy A, Massard C, Soria JC, et al. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–62.
Roche. Roche receives FDA approval for complementary PD-L1 (SP263) biomarker test in urothelial carcinoma [media release]. 2 May 2017. http://www.roche.com.
AstraZeneca. Clinical trials appendix: Q1 2017 results update. 2017. https://www.astrazeneca.com. Accessed 29 May 2017.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Y.Y.Syed is a salaried employee of Adis, Springer SBM.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/C898F06046BCED2D.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
A correction to this article is available online at https://doi.org/10.1007/s40265-017-0826-x.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Syed, Y.Y. Durvalumab: First Global Approval. Drugs 77, 1369–1376 (2017). https://doi.org/10.1007/s40265-017-0782-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0782-5