Abstract
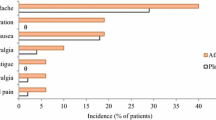
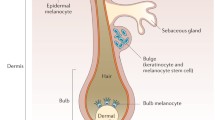
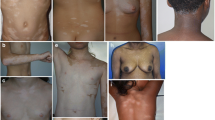
Afamelanotide, the first α-melanocyte-stimulating hormone (MSH) analogue, synthesized in 1980, was broadly investigated in all aspects of pigmentation because its activity and stability were higher than the natural hormone. Afamelanotide binds to the melanocortin-1 receptor (MC1R), and MC1R signaling increases melanin synthesis, induces antioxidant activities, enhances DNA repair processes and modulates inflammation. The loss-of-function variants of the MC1R present in fair-skinned Caucasians are less effectively activated by the natural hormone. Afamelanotide was the first α-MSH analogue to be applied to human volunteers. Ten daily doses of between 0.08 and 0.21 mg/kg in saline injected subcutaneously resulted in long-lasting skin pigmentation and enabled basic pharmacokinetics. Subcutaneous application had full bioavailability, but neither oral nor transdermal application resulted in measurable plasma concentrations or pigmentation response. Two trials in human volunteers showed that neither MC1R variants nor fair skin reduced the afamelanotide-induced increase in skin pigmentation. A controlled-release formulation optimizes administration in man and is effective at a lower dose than the daily saline injections. Promising therapeutic results were published in polymorphic light eruption, erythropoietic protoporphyria (EPP), solar urticaria, Hailey–Hailey disease and vitiligo. In 2014, afamelanotide was approved by the European Medicines Agency for the prevention of phototoxicity in adult patients with EPP. No late effects were reported in volunteers 25 years after the first exposure or after continuous long-term application of up to 8 years in EPP patients, and an immunogenic potential has been excluded. Generally, adverse effects were benign in all trials.

Similar content being viewed by others
References
Malagoli D, Accorsi A, Ottaviani E. The evolution of pro-opiomelanocortin: looking for the invertebrate fingerprints. Peptides. 2011;32:2137–40.
Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–64.
Aroca P, Urabe K, Kobayashi T, Tsukamoto K, Hearing VJ. Melanin biosynthesis patterns following hormonal stimulation. J Biol Chem. 1993;268:25650–5.
del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165–8.
Gibbs S, Murli S, De BG, Mulder A, Mommaas AM, Ponec M. Melanosome capping of keratinocytes in pigmented reconstructed epidermis: effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000;13:458–66.
Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology. 2005;146:532–43.
Osawa M, Egawa G, Mak SS, Moriyama M, Freter R, Yonetani S, et al. Molecular characterization of melanocyte stem cells in their niche. Development. 2005;132:5589–99.
Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602.
Kokot A, Metze D, Mouchet N, Galibert MD, Schiller M, Luger TA, et al. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology. 2009;150:3197–206.
Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126:1966–75.
Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekaro AL, Swope V, Haskell-Luevano C, et al. alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–44.
Bennett DC, Medrano EE. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002;15:242–50.
Hadley ME, Dorr RT. Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides. 2006;27:921–30.
Jiang J, Sharma SD, Nakamura S, Lai JY, Fink JL, Hruby VJ, et al. The melanotropic peptide, [Nle4, D-Phe7] alpha-MSH, stimulates human melanoma tyrosinase activity and inhibits cell proliferation. Pigment Cell Res. 1995;8:314–23.
Eves P, Haycock J, Layton C, Wagner M, Kemp H, Szabo M, et al. Anti-inflammatory and anti-invasive effects of alpha-melanocyte-stimulating hormone in human melanoma cells. Br J Cancer. 2003;89:2004–15.
Zhu N, Eves PC, Katerinaki E, Szabo M, Morandini R, Ghanem G, et al. Melanoma cell attachment, invasion, and integrin expression is upregulated by tumor necrosis factor alpha and suppressed by alpha melanocyte stimulating hormone. J Invest Dermatol. 2002;119:1165–71.
Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–49.
Minder EI. Afamelanotide, an agonistic analog of alpha-melanocyte-stimulating hormone, in dermal phototoxicity of erythropoietic protoporphyria. Expert Opin Investig Drugs. 2010;19:1591–602.
Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, et al. 4-Norleucine, 7-d-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci USA. 1980;77:5754–8.
Hadley ME, Heward CB, Hruby VJ, Sawyer TK, Yang YC. Biological actions of melanocyte-stimulating hormone. Ciba Found Symp. 1981;81:244–62.
Dorr RT, Dawson BV, al-Obeidi F, Hadley ME, Levine N, Hruby VJ. Toxicologic studies of a superpotent alpha-melanotropin, [Nle4, D-Phe7]alpha-MSH. Invest New Drugs. 1988;6:251–8.
Dawson BV, Ford CA, Holloway H, Dorr RT, Johnson P. Administration of melanotropic peptides during gestation in the rodent. Toxicology. 1993;77:91–101.
Castrucci AM, Hadley ME, Sawyer TK, Hruby VJ. Enzymological studies of melanotropins. Comp Biochem Physiol B. 1984;78:519–24.
Peters EM, Tobin DJ, Seidah NG, Schallreuter KU. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol. 2000;114:430–7.
Lerner AB, McGuire JS. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–9.
Levine N, Sheftel SN, Eytan T, Dorr RT, Hadley ME, Weinrach JC, et al. Induction of skin tanning by subcutaneous administration of a potent synthetic melanotropin. JAMA. 1991;266:2730–6.
Dorr RT, Ertl G, Levine N, Brooks C, Bangert JL, Powell MB, et al. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch Dermatol. 2004;140:827–35.
Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA. Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells1. Peptides. 2005;26:1818–24.
Frandberg PA, Muceniece R, Prusis P, Wikberg J, Chhajlani V. Evidence for alternate points of attachment for alpha-MSH and its stereoisomer [Nle4, D-Phe7]-alpha-MSH at the melanocortin-1 receptor. Biochem Biophys Res Commun. 1994;202:1266–71.
Barnetson RS, Ooi TK, Zhuang L, Halliday GM, Reid CM, Walker PC, et al. [Nle4-D-Phe7]-alpha-melanocyte-stimulating hormone significantly increased pigmentation and decreased UV damage in fair-skinned Caucasian volunteers. J Invest Dermatol. 2006;126:1869–78.
Fitzgerald LM, Fryer JL, Dwyer T, Humphrey SM. Effect of MELANOTAN, [Nle(4), D-Phe(7)]-alpha-MSH, on melanin synthesis in humans with MC1R variant alleles. Peptides. 2006;27:388–94.
Luger TA, Scholzen T, Grabbe S. The role of alpha-melanocyte-stimulating hormone in cutaneous biology. J Investig Dermatol Symp Proc. 1997;2:87–93.
Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–72.
Rajora N, Boccoli G, Catania A, Lipton JM. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–5.
Dawson BV, Hadley ME, Levine N, Kreutzfeld KL, Don S, Eytan T, et al. In vitro transdermal delivery of a melanotropic peptide through human skin. J Invest Dermatol. 1990;94:432–5.
Ugwu SO, Blanchard J, Dorr RT, Levine N, Brooks C, Hadley ME, et al. Skin pigmentation and pharmacokinetics of melanotan-I in humans. Biopharm Drug Dispos. 1997;18:259–69.
Bhardwaj R, Blanchard J. Controlled-release delivery system for the alpha-MSH analog melanotan-I using poloxamer 407. J Pharm Sci. 1996;85:915–9.
Bhardwaj R, Hadley ME, Dorr RT, Dvorakova K, Brooks C, Blanchard J. Pharmacologic response of a controlled-release PLGA formulation for the alpha-melanocyte stimulating hormone analog, melanotan-I. Pharm Res. 2000;17:593–9.
Bhardwaj R, Blanchard J. In vitro characterization and in vivo release profile of a poly (d, l-lactide-co-glycolide)-based implant delivery system for the alpha-MSH analog, melanotan-I. Int J Pharm. 1998;170:109–17.
Harms J, Lautenschlager S, Minder CE, Minder EI. An alpha-melanocyte-stimulating hormone analogue in erythropoietic protoporphyria. N Engl J Med. 2009;360:306–7.
Langendonk JG, Balwani M, Anderson KE, Bonkovsky HL, Anstey AV, Bissell DM, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48–59.
Fabrikant J, Touloei K, Brown SM. A review and update on melanocyte stimulating hormone therapy: afamelanotide. J Drugs Dermatol. 2013;12:775–9.
Haylett AK, Nie Z, Brownrigg M, Taylor R, Rhodes LE. Systemic photoprotection in solar urticaria with alpha-melanocyte-stimulating hormone analogue [Nle4-D-Phe7]-alpha-MSH. Br J Dermatol. 2011;164:407–14.
Biolcati G, Aurizi C, Barbieri L, Cialfi S, Screpanti I, Talora C. Efficacy of the melanocortin analogue Nle4-D-Phe7-alpha-melanocyte-stimulating hormone in the treatment of patients with Hailey–Hailey disease. Clin Exp Dermatol. 2014;39:168–75.
Bohm M, Ehrchen J, Luger TA. Beneficial effects of the melanocortin analogue Nle4-D-Phe7-alpha-MSH in acne vulgaris. J Eur Acad Dermatol Venereol. 2014;28:108–11.
Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149:68–73.
Jiang J. Microscopic visualization of melancyte/melanoma melanotropic receptors. Tucson: University of Arizona; 1993.
Smith E, Kiss F, Porter RM, Anstey AV. A review of UVA-mediated photosensitivity disorders. Photochem Photobiol Sci. 2012;11:199–206.
Clinuvel Pharmaceuticals Limited. Clinuvel anounces PLE phase III preliminary results. Clinuvel Pharmaceuticals Ltd; 2009. http://www.clinuvel.com/2009-announcements/item/140-clinuvel-announces-ple-phase-iii-preliminary-results. Accessed 3 Jan 2017.
Schneider-Yin X, Minder EI. Erythropoietic protoporphyria and X-linked dominant protoporphyria. In: Ferreira GC, editor. Porphyrias and sideroblastic anemias. Vol. 29 of the handbook of porphyrin science: 299–328. Series edited by Kadish KM, Simth KM, Guilard R. Singapore: World Scientific Publishing Company; 2013.
Afonso SG, Enriquez DS, Batlle A. Photodynamic and light independent action of 8 to 2 carboxylic free porphyrins on some haem-enzymes. Int J Biochem Cell Biol. 2001;33:1208–14.
Menon IA, Becker MA, Persad SD, Haberman HF. Quantitation of hydrogen peroxide formed during UV-visible irradiation of protoporphyrin, coproporphyrin and uroporphyrin. Clin Chim Acta. 1990;186:375–81.
Lim HW. Mechanisms of phototoxicity in porphyria cutanea tarda and erythropoietic protoporphyria. Immunol Ser. 1989;46:671–85.
Timonen K, Kariniemi AL, Niemi KM, Teppo AM, Tenhunen R, Kauppinen R. Vascular changes in erythropoietic protoporphyria: histopathologic and immunohistochemical study. J Am Acad Dermatol. 2000;43:489–97.
Minder EI, Schneider-Yin X, Steuer J, Bachmann LM. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand). 2009;55:84–97.
Minder EI, Harms J, Lautenschlager S, Schneider-Yin X, Deybach JC, Minder CE. A double-blind, randomized, controlled phase III trial of afamelanotide (an alpha-MSH analogue) in erythropoietic protoporphyria (EPP): preliminary data on a Swiss cohort of patients and a model to determine efficacy in EPP [abstract]. Berzelius Symposium 81: Porphyrins and Porphyrias. Stockholm: 2009.
Langendonk J, Karstens F, SiJbrands E, Hanneken S, Anstey A, Deybach J, et al. Afamelanotide implants effectively reduce pain and prolong sun-tolerance in patients with erythropoietic protoporphyria; results of a phase III, multicenter, double-blind, randomized, placebo-controlled trial. Clin Chem Lab Med. 2013;51:eA12.
Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider-Yin X, Minder EI. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br J Dermatol. 2015;172:1601–12.
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84.
Lim HW, Grimes PE, Lebwohl M. Indications and limitations of afamelanotide for treating vitiligo-reply. JAMA Dermatol. 2015;151:350.
Cialfi S, Oliviero C, Ceccarelli S, Marchese C, Barbieri L, Biolcati G, et al. Complex multipathways alterations and oxidative stress are associated with Hailey–Hailey disease. Br J Dermatol. 2010;162:518–26.
Manca S, Magrelli A, Cialfi S, Lefort K, Ambra R, Alimandi M, et al. Oxidative stress activation of miR-125b is part of the molecular switch for Hailey–Hailey disease manifestation. Exp Dermatol. 2011;20:932–7.
Summary of product characteristics (afamelanotide/Scenesse). 2015.
Ong S, Bowling J. Melanotan-associated melanoma in situ. Australas J Dermatol. 2012;53:301–2.
Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34–6.
Reid C, Fitzgerald T, Fabre A, Kirby B. Atypical melanocytic naevi following melanotan injection. Ir Med J. 2013;106:148–9.
Ellis RA, Kirkham N, Seukeran D. Malignant melanoma in a user of melanotan I. BMJ. 2009;338:b566.
Cardones AR, Grichnik JM. Alpha-melanocyte-stimulating hormone-induced eruptive nevi. Arch Dermatol. 2009;145:441–4.
Cousen P, Colver G, Helbling I. Eruptive melanocytic naevi following melanotan injection. Br J Dermatol. 2009;161:707–8.
Langan EA, Ramlogan D, Jamieson LA, Rhodes LE. Change in moles linked to use of unlicensed “sun tan jab”. BMJ. 2009;338:b277.
Sela M. Antigenicity: some molecular aspects. Science. 1969;166:1365–74.
Mariani M, Bracci L, Presentini R, Nucci D, Neri P, Antoni G. Immunogenicity of a free synthetic peptide: carrier-conjugation enhances antibody affinity for the native protein. Mol Immunol. 1987;24:297–303.
Spichty R, Balimann M, Barman J, Minder EI. A bioassay for detection of neutralizing antibodies against the alpha-melanocyte stimulating hormone analogue afamelanotide in patients with erythropoietic protoporphyria. J Pharm Biomed Analy. 2013;75:192–8.
Lengweiler S, Kreim S, Barman-Aksozen J, Maurer M, Minder EI. Evaluation of the immunogenicity of the synthetic alpha-melanocyte-stimulating hormone (alpha-MSH) analogue afamelanotide ([Nle4-D-Phe7]-alpha-MSH, Scenesse®) in erythropoietic protoporphyria patients by ELISA detecting both anti-afamelanotide and anti-alpha-MSH antibodies. Skin Pharmacol Physiol. 2015;28:103–13.
Fetissov SO, Harro J, Jaanisk M, Jarv A, Podar I, Allik J, et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA. 2005;102:14865–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Elisabeth I. Minder, Jasmin Barman-Aksoezen and Xiaoye Schneider-Yin were partly supported by grants from the Foundation for Scientific Research of Triemli Hospital, the Foundation for Scientific Research of the University of Zurich, the Hartmann–Müller Foundation, and the Velux Foundation. The immunogenicity studies were partly supported by a grant from Clinuvel Pharmaceutical, Melbourne, VIC, Australia.
Conflict of interest
Elisabeth Minder was the principal investigator of two trials of afamelanotide by Clinuvel Pharmaceutical, Melbourne, VIC, Australia. Jasmin Barman-Aksoezen and Xiaoye Schneider-Yin declare that they have no conflicts of interest that might be relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Minder, E.I., Barman-Aksoezen, J. & Schneider-Yin, X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin Pharmacokinet 56, 815–823 (2017). https://doi.org/10.1007/s40262-016-0501-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0501-5