Abstract
Background
Greater drug content requirements for extended-release (ER) opioids necessitate greater protection against dose dumping. Hydrocodone ER employs the CIMA® Abuse-Deterrence Technology platform, which provides resistance against rapid release of the active moiety when the tablet is manipulated or taken with alcohol.
Objective
Assess effects of alcohol on hydrocodone ER pharmacokinetics.
Study Design
Open-label, crossover (January 25–April 30, 2010).
Setting
Single center.
Participants
Forty healthy adults.
Intervention
Subjects received all four treatments in a randomized manner (separated by a minimum 5-day washout): hydrocodone ER 15 mg with 240 mL water and 240 mL orange juice containing 4, 20, and 40 % alcohol in a fasted state. Naltrexone was administered to minimize opioid-related adverse events.
Main Outcome Measure
Effect of alcohol on pharmacokinetics of hydrocodone ER assessed by comparing systemic exposure [maximum plasma drug concentration (C max) and area under the plasma drug concentration-versus-time curve from time 0 to infinity (AUC0–∞)] after administration with alcohol or with water.
Results
Geometric means ratios of hydrocodone ER with 4, 20, and 40 % alcohol relative to water were 1.05, 1.09, and 1.14, respectively, for C max and 1.07, 1.13, and 1.17, respectively, for AUC0–∞. All 90 % confidence intervals for these geometric means ratios fell within the limits of 0.8 and 1.25. Increasing alcohol concentrations did not notably affect systemic exposure but were associated with increased adverse events.
Conclusions
Hydrocodone ER tablets were resistant to dose dumping when administered with alcohol in healthy subjects based on similar systemic exposures observed across all treatments.
Similar content being viewed by others
Alcohol should not be consumed concurrently with opioid therapy. |
Hydrocodone ER formulated with CIMA® ADT appears to be resistant to dose dumping when administered with alcohol, as demonstrated by similar systemic exposures after administration of hydrocodone ER with increasing concentrations of alcohol (4, 20, and 40 %). |
Results suggest that concomitant alcohol consumption has little or no effect on the overall pharmacokinetic profile of hydrocodone ER. |
1 Introduction
Opioids are widely used for the treatment of chronic pain associated with cancer or end-of-life pain and are being increasingly used for chronic non-cancer pain. However, the potential for abuse and misuse remains a concern with opioids [1]. In studies of patients taking opioids for chronic non-cancer pain, approximately 30 % of patients have demonstrated opioid or other substance misuse [2, 3]. The concomitant use of alcohol and opioids is of particular concern, given that a personal or family history of alcohol abuse is strongly predictive of the misuse of opioids for chronic pain [1]. In 2011, of the 606,653 emergency department visits associated with drug misuse or abuse involving drugs and alcohol taken together, 103,730 (17.1 %) were for opioid products [4].
Extended-release (ER) opioid formulations typically contain a higher unit dose of an opioid than immediate-release formulations, and some of those formulations exhibit higher solubility in ethanol than in water. Thus, there is the potential for concomitant use of an ER opioid formulation and alcohol that can lead to rapid release of the opioid (known as dose dumping) and possible overdose [5]. Recently, concerns have been raised in the scientific and regulatory arenas over the potential for alcohol-induced dose dumping with ER opioid formulations [6, 7], and this concern has led to the withdrawal of one controlled-release formulation of hydromorphone [5].
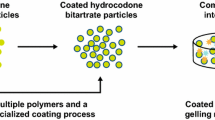
Until recently, hydrocodone was available for the treatment of pain only in immediate-release formulations in combination with other analgesics (e.g. acetaminophen, ibuprofen) [8]. A single-agent, ER formulation of hydrocodone bitartrate (Teva Pharmaceuticals, Frazer, PA, USA) has been developed to provide optimal pain relief with twice-daily dosing. However, given the increased drug content required for an ER formulation and the previously noted concerns related to alcohol-induced dose dumping, this novel hydrocodone ER formulation was developed using CIMA® Abuse Deterrence Technology (ADT) (CIMA Labs, Inc., Brooklyn Park, MN). The CIMA® ADT platform provides resistance against rapid release of the active moiety when the tablet is manipulated or taken with alcohol, potentially reducing product misuse [9]. In the CIMA® ADT process, hydrocodone bitartrate is granulated with a high polymer content and is subsequently coated with a polymeric film to ensure controlled release of hydrocodone over an extended period while limiting the release of active drug when either crushed or exposed to solvents [9]. The polymer-coated granules are compressed into tablets in combination with a gelling matrix that further controls the release of hydrocodone and provides additional resistance to dose dumping when tablets are taken with alcohol.
This study was conducted to assess the effect of increasing concentrations of alcohol on the pharmacokinetics of hydrocodone ER.
2 Methods
This phase 1, single-center, randomized, open-label, crossover study was designed to assess the effect of alcohol on the pharmacokinetics of hydrocodone ER in healthy adults. This study was approved by the institutional review board and conducted in full accordance with the Good Clinical Practice: Consolidated Guidelines approved by the International Conference on Harmonisation [10]. Written informed consent was obtained from all subjects before study participation.
2.1 Subjects
Healthy men and women between 21 and 45 years of age with a body mass index between 20 and 30 kg/m2 and a history of moderate alcohol consumption (7–21 units of alcohol per week; 1 unit = 1 oz hard liquor, 5 oz wine, or 8 oz beer) were eligible to participate. Women were required to be surgically sterile for ≥2 years, 2 years postmenopausal, or using a medically acceptable method of contraception during and for 30 days after the study.
Subjects were excluded if they had any clinically significant uncontrolled medical condition; a history of drug or alcohol abuse or habitual consumption of >21 units of alcohol per week; clinically significant abnormalities in laboratory, electrocardiogram, or physical examination findings; or any disorder that would interfere with medication absorption, distribution, metabolism, or excretion. Subjects were also excluded if they had used any systemic or topical prescription or nonprescription medication (excluding acetaminophen and ibuprofen) within 2 weeks of the first dose of hydrocodone ER; had donated blood (>450 mL) or experienced significant blood loss within 56 days of the first dose of hydrocodone ER; had abnormal heart rate or blood pressure; had consumed food or beverages containing ≥600 mg of caffeine (or ≥5 cups of coffee) per day within 2 weeks of the first dose of hydrocodone ER; had used nicotine products within 12 months; had used topical or oral nicotine cessation products within 3 months of the first dose of hydrocodone ER; or had a history of hypersensitivity or idiosyncratic reaction to hydrocodone, its related compounds, other opioids, or naltrexone.
2.2 Study Design
Healthy subjects were randomized in five different treatment sequences to receive 15 mg of hydrocodone ER with 240 mL of water (under fasting conditions), with 240 mL of orange juice containing 4, 20, or 40 % alcohol (v/v, under fasting conditions), and with 240 mL of water (under fed conditions). Data for hydrocodone ER administered with water under fed conditions are not relevant to the current discussion and are not reported in this paper. The alcohol concentrations were specifically selected to allow for assessment of a broad range of alcohol exposures. Participants received each regimen once, separated by a washout period of 7 days, and had to consume all water or alcohol within 20 min of administration of hydrocodone ER. Subjects took a single, 50-mg tablet of naltrexone 15 and 3 h before and 9 and 21 h after each administration of hydrocodone ER to block opioid receptors and minimize opioid-related adverse events (AEs). Blood samples (3.5 mL) were collected for quantitative blood alcohol concentration measurements immediately before and 1 h after administration of hydrocodone ER in each treatment period. Subjects were withdrawn from the respective treatment period if they vomited within 2.5 h of receiving hydrocodone ER and were permitted to participate in subsequent treatment periods at the discretion of the investigator and medical monitor. Subjects remained in the study center from the day prior to hydrocodone ER administration through 72 h postdose and were asked to return to the study center for a follow-up visit 48–72 h after their last discharge from the center.
2.2.1 Sample Collection and Analytical Methods
Venous blood samples (3 mL) for pharmacokinetics were collected by venipuncture or indwelling catheter approximately 5 min before administration (pre-dose) and 15, 30, and 45 min and 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 18, 24, 30, 36, 48, 60, and 72 h postdose. The samples were collected into K2EDTA tubes and chilled pending centrifugation to separate plasma, which was subsequently stored at approximately −25 °C. The samples were shipped on dry ice to Pharmaceutical Product Development, LLC (Richmond, VA, USA), where they were analyzed for hydrocodone and its active metabolite, hydromorphone, using a validated high-performance liquid chromatography method with tandem mass spectrometric detection. The validated ranges of the method for hydrocodone and hydromorphone were 0.100–100 ng/mL and 0.0500–50.0 ng/mL, respectively.
2.3 Pharmacokinetic Analyses
The pharmacokinetic parameters assessed for hydrocodone ER and its active metabolite, hydromorphone (when feasible), included maximum plasma drug concentration (C max), without interpolation; time to C max (t max); area under the plasma drug concentration-versus-time curve (AUC) from time 0 to 2 h (AUC0–2); AUC from time 0 to 12 h (AUC0–12); AUC from time 0 to infinity (AUC0–∞); percentage extrapolation, calculated as (AUC0–∞–AUC0–t)/(AUC0–∞) × 100, where AUC0–t is the AUC from time 0 to the time of last measurable plasma drug concentration; and terminal elimination half-life (t ½).
2.4 Safety and Tolerability
Safety and tolerability were assessed by evaluating AEs, clinical laboratory data, 12-lead electrocardiogram data, physical examination findings, vital signs (pulse, blood pressure, and respiratory rate), oxyhemoglobin saturation (SpO2), and concomitant medications. Treatment-related AEs were defined as those possibly or probably related to study treatment. AEs were assessed and documented for the duration of the study through to 48–72 h after last discharge from the study center.
2.5 Statistical Methods
Up to 40 healthy subjects were planned to be enrolled in this study, with the intent that approximately 30 subjects would complete the study. This sample size was anticipated to provide at least 80 % power to detect bioequivalence if the intrasubject standard deviation of a natural log-transformed pharmacokinetic parameter was 0.283 or lower.
The safety and pharmacokinetic analysis sets were prespecified. The safety analysis set included all subjects who were randomized to treatment and received at least one dose of hydrocodone ER. The pharmacokinetic analysis set included all subjects in the safety analysis set who had sufficient data for determining pharmacokinetic parameters for the treatment with water and with at least one other treatment.
Pharmacokinetic parameters were summarized with descriptive statistics. The effect of alcohol on the pharmacokinetics of hydrocodone ER was assessed by comparing systemic exposure (C max and AUC) of hydrocodone after administration with alcohol to that with water. The log-transformed values of these parameters were analyzed using the analysis of variance model (ANOVA), which included treatment, treatment sequence, and period as the fixed effects and subject as a random effect (post hoc). The two-sided 90 % CIs for ratios of geometric means of C max and AUC for 4, 20, and 40 % alcohol versus water were calculated. As recommended by industry bioequivalence guidelines [11], if the 90 % CIs were within the limits of 0.8–1.25, then no interaction with alcohol was declared. Box and whisker plots were created post hoc to depict C max and AUC0–∞ data by alcohol concentration.
3 Results
3.1 Subjects
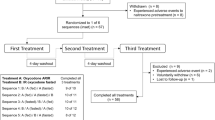
Of the 102 subjects screened, 40 were enrolled and randomized and 39 received at least one dose of hydrocodone ER and were included in the safety analysis set (Fig. 1). Thirty-one subjects completed all four sampling periods, and 30 subjects were included in the pharmacokinetic analysis set. (One subject vomited within 1 h of receiving hydrocodone ER and water and was not eligible for pharmacokinetic comparison.) The majority of participants were men (90 %) and white (87 %), with a median (range) age of 30 (21–44) years and a median (range) body mass index of 25.7 kg/m2 (20.9–29.9 kg/m2). In total, nine subjects discontinued from the study: five for noncompliance to study procedures, three for withdrawal of consent, and one for an AE (vomiting after receiving naltrexone but before receiving the first dose of hydrocodone ER).
3.2 Pharmacokinetics
Administration of hydrocodone ER with 4, 20, or 40 % alcohol did not have a notable effect on the pharmacokinetic profile compared with administration with water. Figures 2 and 3 present the plasma concentration-versus-time profiles through 72 h and through 12 h (planned dosing interval) after administration of hydrocodone ER with water (0 % alcohol) or with 4, 20, or 40 % alcohol, respectively. No appreciable differences in the curves of the mean plasma hydrocodone concentration-versus-time profiles were observed when hydrocodone ER was administered with up to 40 % alcohol. A summary of pharmacokinetic parameters for hydrocodone ER after administration with water and with varying amounts of alcohol is presented in Table 1. Parameters [including measures of systemic exposure (AUC0–∞ range 198.2–228.2 ng·h/mL), C max (range 12.8–14.0 ng/mL), and t max (range 6.0–8.0 h)] were generally comparable among treatment groups. C max and AUC0–∞ data by alcohol concentration are shown in Fig. 4.
Box and whisker plots of C max (a) and AUC0–∞ (b) data by alcohol concentration. Horizontal line represents the median; boxes represent 25th–75th percentiles (Q1–Q3); whiskers represent the minimum and maximum within (Q1–1.5·IQR, Q3 + 1.5·IQR); diamonds represent the mean; circles represent the outliers. AUC 0–∞ area under the plasma hydrocodone concentration-versus-time curve from time 0 to infinity, C max maximum observed plasma hydrocodone concentration, IQR interquartile range, Q quartile
Analysis results based on ANOVA for C max and AUC0–∞ of hydrocodone are presented in Table 2. Systemic exposure was comparable when hydrocodone ER was administered with alcohol (4, 20, or 40 %) or with water. The ratios of the geometric means of hydrocodone exposure with concurrent alcohol relative to water ranged from 1.05 to 1.17. The 90 % CIs for the ratios of geometric means of C max and AUC0–∞ fell within the limits of 0.8 and 1.25 for 4, 20, and 40 % alcohol relative to water.
Systemic exposure to hydromorphone was approximately 1–2 % of that observed for hydrocodone for all regimens.
3.3 Safety and Tolerability
All subjects were administered naltrexone to limit opioid-related AEs. No serious AEs, including deaths, were reported during the study. One subject discontinued from the study due to vomiting; however, this subject was withdrawn after receiving two doses of naltrexone but before receiving the first dose of hydrocodone ER.
In total, 30 subjects (77 %) reported at least one AE. The incidence of AEs increased with increasing concentrations of alcohol (25, 57, and 61 % with 4, 20, and 40 % alcohol, respectively). All AEs were mild or moderate in severity and the most frequently occurring AEs were nausea (46 %), headache (44 %), vomiting (33 %), feeling drunk (28 %), and dizziness (26 %) (Table 3). AEs considered to be treatment-related by the investigator were reported in 22 (56 %) subjects; the most common were nausea (38 %), headache (36 %), and vomiting (26 %). The incidence of treatment-related AEs was greater after 20 % and 40 % alcohol (43 and 48 %, respectively) than after water (22 %) or 4 % alcohol (11 %). AEs were generally consistent with those associated with alcohol consumption.
No clinically significant changes in SpO2, hematology, chemistry, urinalysis, or electrocardiogram results were reported. Eight subjects had clinically significant decreases in systolic blood pressure (≤85 mmHg and decrease from baseline of ≥20 mmHg) and one subject had a clinically significant increase in diastolic blood pressure (≥105 mmHg and increase from baseline of ≥15 mmHg) at least once during the study. None of these blood pressure changes were reported as AEs. Of the 28 subjects with clinically significant respiratory rates (<10 breaths/minute), none of the decreases were reported as AEs or were suggestive of respiratory depression.
4 Discussion
The package inserts of opioid medications include warnings on the potentially additive or synergistic effects of concomitant alcohol use on central nervous system depression, including respiratory depression, hypotension, profound sedation, or coma [12–15]. Furthermore, the updated prescribing labels for morphine sulfate ER capsules (Avinza, Ligand Pharmaceuticals, Inc., San Diego, CA, USA) and oxymorphone hydrochloride ER tablets (Opana ER, King Pharmaceuticals, Inc., Bristol, TN, USA) now include black box warnings advising against the use of alcohol when taking these products because of the risk of dose dumping based on pharmacokinetic interactions [16, 17].
With the increased medication content required for ER formulations, protection against accidental or intentional dose dumping is needed because the rapid release of active medication may increase toxicity [6]. In 2005, the US Food and Drug Administration requested the removal of ER hydromorphone HCl capsules (Palladone, Purdue Pharma L.P., Stamford, CT, USA) from the market after a pharmacokinetic study revealed a dose-dumping effect when the medication was administered concomitantly with alcohol [18]. The average peak hydromorphone concentration increased six-fold when administered with 8 oz of 40 % alcohol compared with water [19]. A pharmacokinetic study of an ER formulation of morphine sulfate and naltrexone hydrochloride showed that coadministration with 40 % alcohol did not affect overall exposure, but did result in a two-fold increase in mean peak plasma concentration of morphine compared with coadministration with water and a reduction of time to reach peak plasma concentration from 9 to 4 h, indicating an earlier release of morphine and a more rapid rate of absorption [20].
Results from the present single-center, randomized, open-label crossover study demonstrate that systemic exposure (C max and AUC) was similar when hydrocodone ER was administered with 240 mL water or orange juice containing 4, 20, or 40 % alcohol, indicating that the risk of alcohol-induced dose dumping associated with the use of hydrocodone ER is low in a fasted state, even at high levels of alcohol exposure (40 %). Furthermore, the comparable findings and lack of marked effect for other pharmacokinetic parameters (e.g. t max, t ½) for subjects receiving alcohol or water suggest that concomitant alcohol consumption has little or no effect on the overall pharmacokinetic profile of hydrocodone ER.
In the current study, hydrocodone ER was generally well tolerated by these subjects who had been administered naltrexone to limit opioid-related AEs. The majority of AEs were mild to moderate in severity, and there were no reports of serious AEs, deaths, or discontinuations due to AEs that were treatment related. When given in combination with hydrocodone ER, the increasing incidence of AEs associated with increasing concentrations of alcohol (4, 20, 40 %) was not unexpected and was likely attributable to the incremental effects of administering the alcohol. Lastly, no clinically significant respiratory depression was reported during the study. Although clinically significant respiratory rates were observed in 28 subjects (<10 breaths/min), data from a separate phase 1 study of hydrocodone ER indicates that clinically significant respiratory rate values were reported both before and after administration of hydrocodone ER and placebo with comparable incidence. As a result, the clinically significant decreased respiratory rate values observed for hydrocodone ER in the current study are not necessarily considered to be related to administration of hydrocodone (data on file, Teva Pharmaceuticals, Frazer, PA, USA).
5 Conclusion
Prescribers should continue to discourage consumption of alcohol during any treatment with opioids. However, results of this analysis provide insights into pharmacokinetic changes that may be anticipated if alcohol is consumed by patients taking hydrocodone ER formulated with CIMA® ADT. Hydrocodone ER formulated with CIMA® ADT appears to be resistant to dose dumping when administered with alcohol, as demonstrated by similar systemic exposures after administration of hydrocodone ER with increasing concentrations of alcohol (4, 20, and 40 %).
References
Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–30.
Chelminski PR, Ives TJ, Felix KM, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5(1):3.
Reid MC, Engles-Horton LL, Weber MB, et al. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173–9.
Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration. http://archive.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Accessed 23 Feb 2015.
Meyer RJ, Hussain AS. FDA’s ACPS meeting, October 2005. Awareness topic: mitigating the risks of ethanol induced dose dumping from oral sustained/controlled release dosage forms. US Food and Drug Administration. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4187B1_01_08-Alcohol-Induced.pdf. Accessed 19 Aug 2015.
Walden M, Nicholls FA, Smith KJ, et al. The effect of ethanol on the release of opioids from oral prolonged-release preparations. Drug Dev Ind Pharm. 2007;33(10):1101–11.
Traynor MJ, Brown MB, Pannala A, et al. Influence of alcohol on the release of tramadol from 24-h controlled-release formulations during in vitro dissolution experiments. Drug Dev Ind Pharm. 2008;34(8):885–9.
The American Society of Health-System Pharmacists. Hydrocodone. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601006.html. Accessed 24 Feb 2015.
CIMA LABS, Inc. OraGuard™: tamper-deterrent, alcohol-resistant extended release technology. 2012. http://www.cimalabs.com/technology/oraguard. Accessed 12 Jan 2015.
International Conference on Harmonisation Working Group. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; June 10, 1996; Washington, DC. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 14 Aug 2015.
Guidance for Industry. Statistical Approaches to Establishing Bioequivalence. US Dept of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070244.pdf. Accessed 24 Feb 2015.
Oxycontin [package insert]. Stamford: Purdue Pharma L.P.; 2013.
Duragesic [package insert]. Titusville: Janssen Pharmaceutica Products, L.P.; 2011.
MS Contin [package insert]. Stamford: Purdue Pharma L.P.; 2009.
OxyIR (oxycodone hydrochloride) immediate-release oral capsules [package insert]. Stamford: Purdue Pharma L.P.; 2010.
Opana ER [package insert]. Chadds Ford: Endo Pharmaceuticals Inc.; 2013.
Avinza [package insert]. Bristol: King Pharmaceuticals, Inc.; 2008.
US Food and Drug Administration. Press release. FDA asks Purdue Pharma to withdraw Palladone for safety reasons. US Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108460.htm. Accessed 19 Aug 2015.
US Food and Drug Administration. Information for healthcare professionals: hydromorphone hydrochloride extended-release capsules (marketed as Palladone). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm129288.htm. Accessed 19 Aug 2015.
Johnson FK, Ciric S, Boudriau S, et al. Effects of alcohol on the pharmacokinetics of morphine sulfate and naltrexone hydrochloride extended release capsules. J Clin Pharmacol. 2012;52(2):747–56.
Acknowledgments
This study was sponsored by Teva Pharmaceuticals (Frazer, PA, USA). Medical writing assistance was provided by Bina Patel, PharmD, CMPP, of Peloton Advantage, LLC, and was funded by Teva Branded Pharmaceutical Products R & D, Inc. (Frazer, PA). Teva provided a full review of this article.
All authors were employees of Teva Pharmaceuticals Industries Ltd at the time of this writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
At the time of this study, MD was an employee of Cephalon, Inc., now a wholly owned subsidiary of Teva Pharmaceuticals (Frazer, PA, USA).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Darwish, M., Bond, M., Yang, R. et al. Assessment of Alcohol-Induced Dose Dumping with a Hydrocodone Bitartrate Extended-Release Tablet Formulated with CIMA® Abuse Deterrence Technology. Clin Drug Investig 35, 645–652 (2015). https://doi.org/10.1007/s40261-015-0324-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0324-4