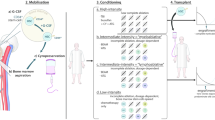
Abstract
Autologous haematopoietic stem cell transplantation (AHSCT) is a treatment option for aggressive forms of multiple sclerosis (MS) that has been derived from haematological indications and repurposed for treatment of refractory autoimmune diseases. In the present review, a search for clinical studies on AHSCT was performed on the PubMed website and ClinicalTrials.gov databases. Papers were selected according to the following criteria: text written in English language, publication date between 2014 and August 2019, and reports including more than five patients. Prospective randomised and uncontrolled trials and retrospective case series were reviewed to examine the safety and efficacy of the procedure. Treatment protocols, pathological data and economic aspects of AHSCT were also succinctly covered. Growing evidence suggests that long-term suppression of inflammatory activity with stabilization or improvement of disability can be achieved in a high proportion of properly selected patients. More sophisticated outcome measures recently adopted, including effect on brain atrophy and disease biomarkers, are giving further insight into the effectiveness of transplant. The risks of the procedure have decreased to levels that can be considered acceptable for treatment of individuals with aggressive forms of MS. Careful selection of patients with an expected good benefit/risk profile, which is maximal when AHSCT is performed in early phases of the disease, and the expertise of transplant centres are critical to the success of treatment. Higher efficacy of AHSCT than with conventional treatments has recently been demonstrated by one randomised trial and further evidence is awaited from ongoing and planned trials comparing AHSCT with the most effective disease-modifying therapeutic agents.



Similar content being viewed by others
References
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622–36. https://doi.org/10.1016/S0140-6736(18)30481-1.
Manouchehrinia A, Beiki O, Hillert J. Clinical course of multiple sclerosis: a nationwide cohort study. Mult Scler. 2017;23(11):1488–95. https://doi.org/10.1177/1352458516681197.
Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25–36. https://doi.org/10.1038/nrneurol.2016.187.
Leray E, Yaouanq J, Le Page E, Coustans M, Laplaud D, Oger J, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133(Pt 7):1900–13. https://doi.org/10.1093/brain/awq076.
Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. https://doi.org/10.1136/bmj.i3518.
Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transpl. 2020;55(2):283–306. https://doi.org/10.1038/s41409-019-0684-0.
Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201(5):805–16. https://doi.org/10.1084/jem.20041679.
Abrahamsson S, Muraro PA. Immune re-education following autologous hematopoietic stem cell transplantation. Autoimmunity. 2008;41(8):577–84. https://doi.org/10.1080/08916930802197081.
Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Investig. 2014;124(3):1168–72. https://doi.org/10.1172/JCI71691.
Karnell FG, Lin D, Motley S, Duhen T, Lim N, Campbell DJ, et al. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin Exp Immunol. 2017;189(3):268–78. https://doi.org/10.1111/cei.12985.
Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136(Pt 9):2888–903. https://doi.org/10.1093/brain/awt182.
de Paula ASA, Malmegrim KC, Panepucci RA, Brum DS, Barreira AA, Carlos Dos Santos A, et al. Autologous haematopoietic stem cell transplantation reduces abnormalities in the expression of immune genes in multiple sclerosis. Clin Sci (Lond). 2015;128(2):111–20. https://doi.org/10.1042/CS20140095.
Arruda LC, Lorenzi JC, Sousa AP, Zanette DL, Palma PV, Panepucci RA, et al. Autologous hematopoietic SCT normalizes miR-16, -155 and -142-3p expression in multiple sclerosis patients. Bone Marrow Transpl. 2015;50(3):380–9. https://doi.org/10.1038/bmt.2014.277.
Collins F, Kazmi M, Muraro PA. Progress and prospects for the use and the understanding of the mode of action of autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2017;13(6):611–22. https://doi.org/10.1080/1744666X.2017.1297232.
Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2301–9. https://doi.org/10.1002/art.10495.
Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri AB, Pieroni F, et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transpl. 2010;45(2):239–48. https://doi.org/10.1038/bmt.2009.127.
Nash RA, Dansey R, Storek J, Georges GE, Bowen JD, Holmberg LA, et al. Epstein–Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transpl. 2003;9(9):583–91.
Moore JJ, Massey JC, Ford CD, Khoo ML, Zaunders JJ, Hendrawan K, et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(5):514–21. https://doi.org/10.1136/jnnp-2018-319446.
Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kurbatova KA, et al. Long-term outcomes of autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis: physician’s and patient’s perspectives. Ann Hematol. 2015;94(7):1149–57. https://doi.org/10.1007/s00277-015-2337-8.
Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–8. https://doi.org/10.1056/NEJMoa1103975.
Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. J Neurol Sci. 2008;274(1–2):42–4. https://doi.org/10.1016/j.jns.2008.06.032.
Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol. 1989;24(6):386–90. https://doi.org/10.1007/bf00257448.
Egorin MJ, Kaplan RS, Salcman M, Aisner J, Colvin M, Wiernik PH, et al. Cyclophosphamide plasma and cerebrospinal fluid kinetics with and without dimethyl sulfoxide. Clin Pharmacol Ther. 1982;32(1):122–8. https://doi.org/10.1038/clpt.1982.135.
Prodduturi P, Bierman PJ. Current and emerging pharmacotherapies for primary CNS lymphoma. Clin Med Insights Oncol. 2012;6:219–31. https://doi.org/10.4137/CMO.S7752.
Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–305. https://doi.org/10.1200/jco.2006.09.9861.
Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85(10):1116–21. https://doi.org/10.1136/jnnp-2013-307207.
Rush CA, Atkins HL, Freedman MS. Autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Cold Spring Harb Perspect Med. 2019. https://doi.org/10.1101/cshperspect.a029082.
Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17(5):467–80. https://doi.org/10.1016/S1474-4422(18)30040-1.
Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. https://doi.org/10.1016/j.msard.2017.01.006.
Frau J, Carai M, Coghe G, Fenu G, Lorefice L, La Nasa G, et al. Long-term follow-up more than 10 years after HSCT: a monocentric experience. J Neurol. 2018;265(2):410–6. https://doi.org/10.1007/s00415-017-8718-2.
Guida M, Castaldi MA, Rosamilio R, Giudice V, Orio F, Selleri C. Reproductive issues in patients undergoing hematopoietic stem cell transplantation: an update. J Ovarian Res. 2016;9(1):72. https://doi.org/10.1186/s13048-016-0279-y.
Snarski E, Snowden JA, Oliveira MC, Simoes B, Badoglio M, Carlson K, et al. Onset and outcome of pregnancy after autologous haematopoietic SCT (AHSCT) for autoimmune diseases: a retrospective study of the EBMT autoimmune diseases working party (ADWP). Bone Marrow Transpl. 2015;50(2):216–20. https://doi.org/10.1038/bmt.2014.248.
Massenkeil G, Alexander T, Rosen O, Dorken B, Burmester G, Radbruch A, et al. Long-term follow-up of fertility and pregnancy in autoimmune diseases after autologous haematopoietic stem cell transplantation. Rheumatol Int. 2016;36(11):1563–8. https://doi.org/10.1007/s00296-016-3531-2.
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74(4):459–69. https://doi.org/10.1001/jamaneurol.2016.5867.
Daikeler T, Labopin M, Di Gioia M, Abinun M, Alexander T, Miniati I, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118(6):1693–8. https://doi.org/10.1182/blood-2011-02-336156.
Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388(10044):576–85. https://doi.org/10.1016/S0140-6736(16)30169-6.
Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313(3):275–84. https://doi.org/10.1001/jama.2014.17986.
Casanova B, Jarque I, Gascon F, Hernandez-Boluda JC, Perez-Miralles F, de la Rubia J, et al. Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis. Neurol Sci. 2017;38(7):1213–21. https://doi.org/10.1007/s10072-017-2933-6.
Danylesko I, Shimoni A. Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol. 2018;19(2):9. https://doi.org/10.1007/s11864-018-0528-y.
Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs. 2018;32(10):939–49. https://doi.org/10.1007/s40263-018-0564-y.
Walker LA, Berard JA, Atkins HL, Bowman M, Lee H, Freedman MS. Cognitive change and neuroimaging following immunoablative therapy and hematopoietic stem cell transplantation in multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2014;3(1):129–35. https://doi.org/10.1016/j.msard.2013.05.001.
Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13(7):391–405. https://doi.org/10.1038/nrneurol.2017.81.
Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015;84(10):981–8. https://doi.org/10.1212/wnl.0000000000001329.
Currò D, Vuolo L, Gualandi F, Bacigalupo A, Roccatagliata L, Capello E, et al. Low intensity lympho-ablative regimen followed by autologous hematopoietic stem cell transplantation in severe forms of multiple sclerosis: a MRI-based clinical study. Mult Scler. 2015;21(11):1423–30. https://doi.org/10.1177/1352458514564484.
Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165–74. https://doi.org/10.1001/jama.2018.18743.
Tolf A, Fagius J, Carlson K, Akerfeldt T, Granberg T, Larsson EM, et al. Sustained remission in multiple sclerosis after hematopoietic stem cell transplantation. Acta Neurol Scand. 2019;140(5):320–7. https://doi.org/10.1111/ane.13147.
Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Steinmiller KC, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88(9):842–52. https://doi.org/10.1212/WNL.0000000000003660.
Ge F, Lin H, Li Z, Chang T. Efficacy and safety of autologous hematopoietic stem-cell transplantation in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2019;40(3):479–87. https://doi.org/10.1007/s10072-018-3670-1.
Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology. 2017;88(22):2115–22. https://doi.org/10.1212/WNL.0000000000003987.
Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1(27):2742–55. https://doi.org/10.1182/bloodadvances.2017010041.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. https://doi.org/10.1056/NEJMoa044397.
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–23. https://doi.org/10.1056/NEJMoa044396.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28. https://doi.org/10.1016/S0140-6736(12)61769-3.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39. https://doi.org/10.1016/S0140-6736(12)61768-1.
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34. https://doi.org/10.1056/NEJMoa1601277.
Mancardi GL, Saccardi R, Filippi M, Gualandi F, Murialdo A, Inglese M, et al. Autologous hematopoietic stem cell transplantation suppresses Gd-enhanced MRI activity in MS. Neurology. 2001;57(1):62–8. https://doi.org/10.1212/wnl.57.1.62.
Filippi M, Rovaris M, Capra R, Gasperini C, Yousry TA, Sormani MP, et al. A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis. Implications for phase II clinical trials. Brain. 1998;121(Pt 10):2011–20. https://doi.org/10.1093/brain/121.10.2011.
Sormani MP, Muraro PA, Saccardi R, Mancardi G. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler. 2017;23(2):201–4. https://doi.org/10.1177/1352458516645670.
Popescu V, Agosta F, Hulst HE, Sluimer IC, Knol DL, Sormani MP, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(10):1082–91. https://doi.org/10.1136/jnnp-2012-304094.
De Stefano N, Stromillo ML, Giorgio A, Bartolozzi ML, Battaglini M, Baldini M, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(1):93–9. https://doi.org/10.1136/jnnp-2014-309903.
Dwyer MG, Hagemeier J, Bergsland N, Horakova D, Korn JR, Khan N, et al. Establishing pathological cut-offs for lateral ventricular volume expansion rates. Neuroimage Clin. 2018;18:494–501. https://doi.org/10.1016/j.nicl.2018.02.009.
Martola J, Stawiarz L, Fredrikson S, Hillert J, Bergstrom J, Flodmark O, et al. Progression of non-age-related callosal brain atrophy in multiple sclerosis: a 9-year longitudinal MRI study representing four decades of disease development. J Neurol Neurosurg Psychiatry. 2007;78(4):375–80. https://doi.org/10.1136/jnnp.2006.106690.
De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–76. https://doi.org/10.1212/WNL.0b013e3181e24136.
Chen JT, Collins DL, Atkins HL, Freedman MS, Galal A, Arnold DL, et al. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology. 2006;66(12):1935–7. https://doi.org/10.1212/01.wnl.0000219816.44094.f8.
Rocca MA, Mondria T, Valsasina P, Sormani MP, Flach ZH, Te Boekhorst PA, et al. A three-year study of brain atrophy after autologous hematopoietic stem cell transplantation in rapidly evolving secondary progressive multiple sclerosis. AJNR Am J Neuroradiol. 2007;28(9):1659–61. https://doi.org/10.3174/ajnr.A0644.
Lee H, Narayanan S, Brown RA, Chen JT, Atkins HL, Freedman MS, et al. Brain atrophy after bone marrow transplantation for treatment of multiple sclerosis. Mult Scler. 2017;23(3):420–31. https://doi.org/10.1177/1352458516650992.
Mondria T, Lamers CH, te Boekhorst PA, Gratama JW, Hintzen RQ. Bone-marrow transplantation fails to halt intrathecal lymphocyte activation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79(9):1013–5. https://doi.org/10.1136/jnnp.2007.133520.
Larsson D, Akerfeldt T, Carlson K, Burman J. Intrathecal immunoglobulins and neurofilament light after autologous haematopoietic stem cell transplantation for multiple sclerosis. Mult Scler. 2019. https://doi.org/10.1177/1352458519863983.
Rejdak K, Stelmasiak Z, Grieb P. Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult Scler Relat Disord. 2019;27:117–20. https://doi.org/10.1016/j.msard.2018.10.006.
Mancuso R, Franciotta D, Rovaris M, Caputo D, Sala A, Hernis A, et al. Effects of natalizumab on oligoclonal bands in the cerebrospinal fluid of multiple sclerosis patients: a longitudinal study. Mult Scler. 2014;20(14):1900–3. https://doi.org/10.1177/1352458514538111.
Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019. https://doi.org/10.1001/jamaneurol.2019.1534.
Novakova L, Zetterberg H, Sundstrom P, Axelsson M, Khademi M, Gunnarsson M, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–7. https://doi.org/10.1212/WNL.0000000000004683.
Thebault S, Tessier D, Lee H, Bowman M, Bar-Or A, Arnold DL, et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e598. https://doi.org/10.1212/NXI.0000000000000598.
Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, et al. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007;130(Pt 5):1254–62. https://doi.org/10.1093/brain/awl370.
Wundes A, Bowen JD, Kraft GH, Maravilla KR, McLaughlin B, von Geldern G, et al. Brain pathology of a patient 7 years after autologous hematopoietic stem cell transplantation for multiple sclerosis. J Neurol Sci. 2017;373:339–41. https://doi.org/10.1016/j.jns.2017.01.016.
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8(3):254–60. https://doi.org/10.1016/S1474-4422(09)70021-3.
Oturai AB, Koch-Henriksen N, Petersen T, Jensen PE, Sellebjerg F, Sorensen PS. Efficacy of natalizumab in multiple sclerosis patients with high disease activity: a Danish nationwide study. Eur J Neurol. 2009;16(3):420–3. https://doi.org/10.1111/j.1468-1331.2008.02517.x.
Maximizing Outcome of Multiple Sclerosis Transplantation (MOST) [ClinicalTrials.gov identifier NCT03342638]. National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03342638?term=autologous+hematopoietic+stem+cells+transplantation&recrs=abd&cond=Multiple+Sclerosis&rank=4. Accessed 20 Aug 2019.
Batcheller L, Baker D. Cost of disease modifying therapies for multiple sclerosis: is front-loading the answer? J Neurol Sci. 2019;404:19–28. https://doi.org/10.1016/j.jns.2019.07.009.
Kobelt G, Berg J, Lindgren P, Jonsson B, Stawiarz L, Hillert J. Modeling the cost-effectiveness of a new treatment for MS (natalizumab) compared with current standard practice in Sweden. Mult Scler. 2008;14(5):679–90. https://doi.org/10.1177/1352458507086667.
Ernstsson O, Gyllensten H, Alexanderson K, Tinghog P, Friberg E, Norlund A. Cost of illness of multiple sclerosis—a systematic review. PLoS One. 2016;11(7):e0159129. https://doi.org/10.1371/journal.pone.0159129.
Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–36. https://doi.org/10.1177/1352458517694432.
Tinelli M, Kanavos P, Efthymiadou O, Visintin E, Grimaccia F, Mossman J. Using IMPrESS to guide policy change in multiple sclerosis. Mult Scler. 2018;24(9):1251–5. https://doi.org/10.1177/1352458517737388.
Tappenden P, Saccardi R, Confavreux C, Sharrack B, Muraro PA, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for secondary progressive multiple sclerosis: an exploratory cost-effectiveness analysis. Bone Marrow Transpl. 2010;45(6):1014–21. https://doi.org/10.1038/bmt.2009.305.
Burman J, Kirgizov K, Carlson K, Badoglio M, Mancardi GL, De Luca G, et al. Autologous hematopoietic stem cell transplantation for pediatric multiple sclerosis: a registry-based study of the Autoimmune Diseases Working Party (ADWP) and Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2017;52(8):1133–7. https://doi.org/10.1038/bmt.2017.40.
Borgmann-Staudt A, Rendtorff R, Reinmuth S, Hohmann C, Keil T, Schuster FR, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transpl. 2012;47(2):271–6. https://doi.org/10.1038/bmt.2011.78.
Vatanen A, Wilhelmsson M, Borgstrom B, Gustafsson B, Taskinen M, Saarinen-Pihkala UM, et al. Ovarian function after allogeneic hematopoietic stem cell transplantation in childhood and adolescence. Eur J Endocrinol. 2014;170(2):211–8. https://doi.org/10.1530/EJE-13-0694.
Dvorak CC, Gracia CR, Sanders JE, Cheng EY, Baker KS, Pulsipher MA, et al. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, and reproductive risks. Biol Blood Marrow Transpl. 2011;17(12):1725–38. https://doi.org/10.1016/j.bbmt.2011.10.006.
Jadoul P, Anckaert E, Dewandeleer A, Steffens M, Dolmans MM, Vermylen C, et al. Clinical and biologic evaluation of ovarian function in women treated by bone marrow transplantation for various indications during childhood or adolescence. Fertil Steril. 2011;96(1):126–133.e3. https://doi.org/10.1016/j.fertnstert.2011.03.108.
Harding KE, Liang K, Cossburn MD, Ingram G, Hirst CL, Pickersgill TP, et al. Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):141–7. https://doi.org/10.1136/jnnp-2012-303996.
Burman J, Tolf A, Hagglund H, Askmark H. Autologous haematopoietic stem cell transplantation for neurological diseases. J Neurol Neurosurg Psychiatry. 2018;89(2):147–55. https://doi.org/10.1136/jnnp-2017-316271.
Daumer M, Griffith LM, Meister W, Nash RA, Wolinsky JS. Survival, and time to an advanced disease state or progression, of untreated patients with moderately severe multiple sclerosis in a multicenter observational database: relevance for design of a clinical trial for high dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation. Mult Scler. 2006;12(2):174–9. https://doi.org/10.1191/135248506ms1256oa.
Hirst C, Ingram G, Swingler R, Compston DA, Pickersgill T, Robertson NP. Change in disability in patients with multiple sclerosis: a 20-year prospective population-based analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1137–43. https://doi.org/10.1136/jnnp.2007.133785.
Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253(1):98–108. https://doi.org/10.1007/s00415-005-0934-5.
Mariottini A, Innocenti C, Forci B, Magnani E, Mechi C, Barilaro A, et al. Safety and efficacy of autologous hematopoietic stem-cell transplantation following natalizumab discontinuation in aggressive multiple sclerosis. Eur J Neurol. 2019;26(4):624–30. https://doi.org/10.1111/ene.13866.
Cohen JA, Baldassari LE, Atkins HL, Bowen JD, Bredeson C, Carpenter PA, et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25(5):845–54. https://doi.org/10.1016/j.bbmt.2019.02.014.
Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2012;47(6):770–90. https://doi.org/10.1038/bmt.2011.185.
Acknowledgements
We are grateful for support from the National Institute of Health Research (NIHR) (EME Project: 16/126/26 to PM) and the NIHR Biomedical Research Centre funding scheme to Imperial College London.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. A. Mariottini discloses personal fees from Merck-Serono and non-financial support from Biogen Idec, Teva and Novartis. Dr. E. De Matteis reports no conflict of interest. Prof. Paolo A. Muraro discloses travel support and speaker honoraria from unrestricted educational activities organised by Novartis, Bayer HealthCare, Bayer Pharma, Biogen Idec, Merck-Serono and Sanofi Aventis.
Funding
The present work was supported by the National Institute of Health Research (EME Project: 16/126/26 to PM) and the NIHR Biomedical Research Centre funding scheme to Imperial College London.
Rights and permissions
About this article
Cite this article
Mariottini, A., De Matteis, E. & Muraro, P.A. Haematopoietic Stem Cell Transplantation for Multiple Sclerosis: Current Status. BioDrugs 34, 307–325 (2020). https://doi.org/10.1007/s40259-020-00414-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-020-00414-1