Abstract
Objective
Compared to traditional drugs, specialty drugs tend to be indicated for lower prevalence diseases. Our objective was to compare the potential population health benefits associated with specialty and traditional drugs in the year following product approval.
Methods
First, we created a dataset of estimates of incremental quality-adjusted life-year (QALY) gains and incremental life-year (LY) gains for US FDA-approved drugs (1999–2011) compared to standard of care at the time of approval identified from a literature search. Second, we categorized each drug as specialty or traditional. Third, for each drug we identified estimates of US disease prevalence for each pertinent indication. Fourth, in order to conservatively estimate the potential population health gains associated with each new drug in the year following its approval we multiplied the health gain estimate by 10% of the identified prevalence. Fifth, we used Mann–Whitney U tests to compare the population health gains for specialty and traditional drugs.
Results
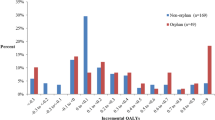
We identified QALY gain estimates for 101 drugs, including 56 specialty drugs, and LY gain estimates for 50 drugs, including 34 specialty drugs. The median estimated population QALY gain in the year following approval for specialty drugs was 4200 (IQR = 27,000) and for traditional drugs was 694 (IQR = 24,400) (p = 0.245). The median estimated population LY gain in the year following approval for specialty drugs was 7250 (IQR = 39,200) and for traditional drugs was 2500 (IQR = 58,200) (p = 0.752).
Conclusions
Despite often being indicated for diseases of lower prevalence, we found a trend towards specialty drugs offering larger potential population health gains than traditional drugs, particularly when measured in terms of QALYs.



Similar content being viewed by others
References
Gleason PP, Alexander GC, Starner CI, Ritter ST, Van Houten HK, Gunderson BW, et al. Health plan utilization and costs of specialty drugs within 4 chronic conditions. J Manag Care Pharm. 2013;19(7):542–8.
Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood). 2006;25(5):1319–31.
Sullivan SD. The promise of specialty pharmaceuticals: are they worth the price? J Manag Care Pharm. 2008;14(4 Suppl):S3–6.
Schilling B. Specialty Drug Costs Poised to Skyrocket but Many Employers Have Yet to Take Note. The Commonwealth Fund. April 11, 2012. http://www.commonwealthfund.org/publications/newsletters/purchasing-high-performance/2012/april-11-2012/featured-articles/specialty-drug-costs-poised-to-skyrocket. Accessed 5 Oct 2016.
Chambers JD, Thorat T, Pyo J, Chenoweth M, Neumann PJ. Despite high costs, specialty drugs may offer value for money comparable to that of traditional drugs. Health Aff (Millwood). 2014;33(10):1751–60.
Cutler DM, McClellan M. Is technological change in medicine worth it? Health Aff (Millwood). 2001;20(5):11–29.
Cutler DM, Long G, Berndt ER, Royer J, Fournier AA, Sasser A, et al. The value of antihypertensive drugs: a perspective on medical innovation. Health Aff (Millwood). 2007;26(1):97–110.
Lichtenberg FR. The quality of medical care, behavioral risk factors, and longevity growth. Int J Health Care Finance Econ. 2011;11(1):1–34.
Lichtenberg FR. Contribution of pharmaceutical innovation to longevity growth in Germany and France, 2001–7. Pharmacoeconomics. 2012;30(3):197–211.
Lichtenberg FR. The effect of using newer drugs on admissions of elderly Americans to hospitals and nursing homes: state-level evidence from 1997 to 2003. Pharmacoeconomics. 2006;24(Suppl 3):5–25.
Lichtenberg FR. The impact of pharmaceutical innovation on premature cancer mortality in Switzerland, 1995–2012. Eur J Health Econ. 2016;17(7):833–54.
Lichtenberg FR. The impact of new (orphan) drug approvals on premature mortality from rare diseases in the United States and France, 1999–2007. Eur J Health Econ. 2013;14(1):41–56.
Lichtenberg FR. Has medical innovation reduced cancer mortality? CESifo Econ Stud. 2014;60(1):135–77.
Chambers JD, Thorat T, Chenoweth MD, Neumann PJ. Fast-tracking innovation: a scorecard for the FDA’s expedited review process. Value Health. 2015;18(7):A517.
Caremark CVS. Comprehensive specialty pharmacy drug list. Woonsocket: CVS Caremark; 2015.
Scripts Express. Specialty drug list by disease state. St. Louis: Express Scripts Holding Company; 2015.
Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703.
Garattini L, Koleva D, Casadei G. Modeling in pharmacoeconomic studies: funding sources and outcomes. Int J Technol Assess Health Care. 2010;26(3):330–3.
Centers for Disease Control and Prevention. Behavioral risk factor surveillance system—prevalence data & data analysis tools. http://www.cdcgov/brfss/data_tools.htm. Accessed 5 Oct 2016.
National Cancer Institute—Surveillance EaERP. SEER Cancer Statistics Review (CSR) 1975–2012. http://seer.cancer.gov/csr/1975_2012/. Accessed 5 Oct 2016.
The United States Census Bureau. Population and Housing Unit Estimates. http://www.census gov/popest/index.html. Accessed 5 Oct 2016.
Board of Governors of the Federal Reserve System. Foreign exchange rates—H.10. 10-3-2016. Accessed 5 Oct 2016.
Bureau of Labor Statistics. Consumer Price Index. Department of Labor. http://www.bls.gov/cpi/. Accessed 5 Oct 2016.
Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–12.
Garjon FJ, Azparren A, Vergara I, Azaola B, Loayssa JR. Adoption of new drugs by physicians: a survival analysis. BMC Health Serv Res. 2012;12:56.
Chambers JD, Thorat T, Pyo J, Neumann PJ. The lag from FDA approval to published cost-utility evidence. Expert Rev Pharmacoecon Outcomes Res. 2015;15(3):399–402.
Wisloff T, Hagen G, Hamidi V, Movik E, Klemp M, Olsen JA. Estimating QALY gains in applied studies: a review of cost-utility analyses published in 2010. Pharmacoeconomics. 2014;32(4):367–75.
Neumann PJ, Greenberg D, Olchanski NV, Stone PW, Rosen AB. Growth and quality of the cost-utility literature, 1976–2001. Value Health. 2005;8(1):3–9.
Neumann PJ, Fang CH, Cohen JT. 30 years of pharmaceutical cost-utility analyses: growth, diversity and methodological improvement. Pharmacoeconomics. 2009;27(10):861–72.
Coast J. Is economic evaluation in touch with society’s health values? BMJ. 2004;329(7476):1233–6.
Garau M, Shah KK, Mason AR, Wang Q, Towse A, Drummond MF. Using QALYs in cancer: a review of the methodological limitations. Pharmacoeconomics. 2011;29(8):673–85.
Williams A. QALYS and ethics: a health economist’s perspective. Soc Sci Med. 1996;43(12):1795–804.
Bach PB, Pearson SD. Payer and policy maker steps to support value-based pricing for drugs. JAMA. 2015;314(23):2503–4.
Neumann PJ, Cohen JT. Measuring the value of prescription drugs. N Engl J Med. 2015;373(27):2595–7.
Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–19.
Schulman KA, Balu S, Reed SD. Specialty pharmaceuticals for hyperlipidemia-impact on insurance premiums. N Engl J Med. 2015;373(17):1591–3.
Brennan TA, Wilson JM. The special case of gene therapy pricing. Nat Biotechnol. 2014;32(9):874–6.
Gottlieb S, Carino T. Establishing new payment provisions for the high cost of curing disease. http://www.aei.org/publication/establishing-new-payment-provisions-for-the-high-cost-of-curing-disease. Accessed 5 Oct 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
The study was funded by Pfizer. Researchers at Tufts Medical Center retained full control over question formulation, study selection, data extraction, data analyses, and interpretation of results.
Conflicts of interest
JC, TT, CW, MS, and PJN report receiving grant funding from Pfizer. PS and SKB report employment, including stock ownership/options, by Pfizer.
Author contributions
James Chambers: concept and design, analysis and interpretation of data, drafting of the manuscript, obtaining funding. Teja Thorat: statistical analysis, analysis and interpretation of data, drafting of the manuscript. Mark Salem: acquisition of data, administrative support, analysis and interpretation of data, drafting of the manuscript. Colby Wilkinson: acquisition of data, analysis and interpretation of data, statistical analysis. Prasun Subedi: concept and design, critical revision of the manuscript for important intellectual content, analysis and interpretation of data. Sachin Kamal-Bahl: concept and design, critical revision of the manuscript for important intellectual content, analysis and interpretation of data. Peter Neumann: supervision, obtaining funding, critical revision of the manuscript for important intellectual content.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chambers, J.D., Thorat, T., Wilkinson, C.L. et al. Estimating Population Health Benefits Associated with Specialty and Traditional Drugs in the Year Following Product Approval. Appl Health Econ Health Policy 15, 227–235 (2017). https://doi.org/10.1007/s40258-016-0291-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0291-9