Abstract
Background
Patients with psoriasis who have an inadequate response to one biologic may benefit from switching to a new biologic, such as ixekizumab, a high affinity monoclonal antibody that selectively targets interleukin (IL)-17A.
Objective
Our aim was to assess the response to ixekizumab in patients with moderate-to-severe plaque psoriasis who did not respond adequately to etanercept using a post-hoc analysis in two phase III studies.
Methods
For the subanalyses in two phase III trials (UNCOVER-2 and -3), non-response was defined by either failure to have a static physician global assessment (sPGA) of 0/1 in UNCOVER-2 or failure to have at least 75% improvement in psoriasis area and severity index (PASI 75) in UNCOVER-3 at Week 12 of each study. Non-responders treated with twice-weekly etanercept 50 mg in the first 12 weeks received two injections of placebo at Week 12 (4-week wash-out period), followed by ixekizumab every 4 weeks (Q4W) for Weeks 16–60. Non-responders to placebo in the first 12 weeks were administered ixekizumab 160 mg at Week 12, followed by ixekizumab Q4W for Weeks 16–60.
Results
After switching to ixekizumab Q4W, a substantial proportion of patients with moderate-to-severe psoriasis who did not respond to etanercept experienced rapid and durable improvement in all efficacy evaluations. Among sPGA 0/1 (UNCOVER-2) and PASI 75 (UNCOVER-3) non-responders to etanercept, 73.0% achieved sPGA 0/1 and 78.2% achieved PASI 75, respectively, after 12 weeks of ixekizumab treatment. Safety profiles in patients switched from etanercept to ixekizumab were similar to those in patients switched from placebo to ixekizumab.
Conclusion
Patients who were non-responders to etanercept after 12 weeks, as defined by failure to meet sPGA 0/1 (UNCOVER-2) or PASI 75 (UNCOVER-3), achieved high levels of response 12 weeks after switching to ixekizumab.
Studies are registered with ClinicalTrials.gov (NCT01597245 and NCT01646177).
Similar content being viewed by others
Alternative therapies are needed for patients with psoriasis who must discontinue a tumor necrosis factor alpha (TNF-α) inhibitor due to limited efficacy, loss of efficacy, or adverse reactions. |
In patients switched to ixekizumab following non-response to etanercept, a TNF-α inhibitor, we observed a favorable response in the majority of patients. |
In both UNCOVER-2 and -3, safety profiles were comparable in placebo non-responders and etanercept non-responders after switching to ixekizumab. |
1 Introduction
There are several emerging biologic therapies for the treatment of psoriasis, providing patients with more options for the management of this chronic and at times debilitating disease [1–4]. Availability of new agents raises the question of how prior treatment might affect future therapies. Patients who do not respond optimally to one biologic therapy may benefit from switching to a biologic that acts through a different mechanism. Indeed, in the ACCEPT trial, psoriasis area and severity index (PASI) response rates of patients who were non-responders to etanercept during the induction period improved after switching to ustekinumab, even though response rates after 12 weeks were lower among these patients compared with those given ustekinumab during the induction period [5]. On the other hand, a Danish observational study found no association between prior treatment with tumor necrosis factor alpha (TNF-α) inhibitors and response to subsequent therapy with ustekinumab [6].
Ixekizumab is a high affinity monoclonal antibody that selectively targets interleukin (IL)-17A. This drug has previously been reported to have rapid and lasting efficacy in the treatment of moderate-to-severe plaque psoriasis in three phase III clinical trials (UNCOVER-1, -2, and -3) [1, 2]. Two of these trials (UNCOVER-2 and -3) included an etanercept treatment arm during the induction period (Weeks 0–12). In this post-hoc subanalysis of UNCOVER-2 and -3 trials, we examined the safety and efficacy of ixekizumab in patients switched to ixekizumab every 4 weeks (Q4W) following non-response (NR) to 12 weeks of treatment with etanercept or placebo. For completeness of the analysis in trials with differing study designs, we looked at two different criteria for defining non-responders: failure to reach the static physician global assessment (sPGA) 0/1 (UNCOVER-2) or failure to reach 75% improvement in PASI (PASI 75; UNCOVER-3).
2 Methods
2.1 Patients
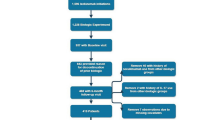
An investigational review board at each site approved study protocols and informed consent forms, and all patients signed informed consent prior to undergoing study-related procedures. UNCOVER-2 (NCT01597245) was registered on May 10, 2012 and UNCOVER-3 (NCT01646177) was registered on July 18, 2012 with ClinicalTrials.gov. Complete patient eligibility criteria for the UNCOVER-2 and -3 trials have been previously described [1]. Briefly, patients aged 18 years or older were eligible with a confirmed diagnosis of chronic plaque psoriasis at least 6 months prior to baseline (randomization), at least a moderate disease severity as measured by clinician-rated measure of sPGA score ≥3, at least 10% body surface area (BSA) involvement, and PASI score ≥12 at both screening and baseline visits. Patients with prior exposure to etanercept were excluded from these studies.
2.2 Study Design and Treatment Regimens
2.2.1 Induction Period (UNCOVER-2 and -3): Weeks 0–12
During the 12-week placebo- and active-controlled period in each of these phase III trials, patients were randomized at a 2:2:2:1 ratio stratified by center to one of the following treatment groups: ixekizumab 80 mg every 2 weeks (Q2W) or Q4W following a starting dose of 160 mg, etanercept 50 mg twice weekly, or placebo.
2.2.2 Weeks 12–60 (UNCOVER-2)
In UNCOVER-2, failure to meet sPGA 0/1 at Week 12 was the pre-specified criterion defining non-response to treatment. All sPGA 0/1 non-responders, regardless of induction treatment group, were switched to ixekizumab 80 mg Q4W. Therefore, UNCOVER-2 data support the analysis of patients who were non-responders to etanercept based on sPGA definition. All patients who received etanercept in the induction period and did not achieve sPGA 0/1 at Week 12 underwent a 4-week washout period where placebo injections were given at Week 12, and patients were started on ixekizumab 80 mg Q4W at Week 16. All patients who received placebo in the induction period who did not achieve sPGA 0/1 at Week 12 were given ixekizumab 160 mg at Week 12, and ixekizumab 80 mg Q4W starting at Week 16.
2.2.3 Weeks 12–60 (UNCOVER-3)
In UNCOVER-3, all patients, regardless of induction treatment group and sPGA response at Week 12, were switched to ixekizumab 80 mg Q4W. Therefore, UNCOVER-3 data support the analysis of patients who were non-responders to etanercept based on PASI 75 definition. All patients who received etanercept in the induction period (regardless of response at Week 12) underwent a 4-week washout period where placebo injections were given at Week 12, followed by ixekizumab Q4W starting at Week 16. All patients who received placebo in the induction period were given ixekizumab160 mg at Week 12 and ixekizumab 80 mg Q4W starting at Week 16. Safety data for UNCOVER-3 is based on the data reported at the time of the Week 60 interim database lock; therefore, some events occurred beyond 60 weeks.
2.3 Statistical Analyses
For the analyses described here, we looked at two definitions of NR. In UNCOVER-2, the pre-specified definition of NR based on FDA guidance was failure to meet sPGA 0/1 at Week 12. In a post-hoc subanalysis of UNCOVER-3, NR was defined as failure to achieve PASI 75 by Week 12.
Non-responder imputation (NRI) was used for categorical variables for missing data. Change from baseline PASI and percent improvement in PASI used last observation carried forward (LOCF), and treatment comparisons in the induction period for these variables were done using an analysis of covariance model, including treatment, pooled center, and baseline (Week 0) PASI value in the model.
Safety analyses were conducted on all patients who received at least one dose of assigned study treatment during the study period. An adverse event is considered a treatment-emergent adverse event if it first occurs or worsens following the start of treatment during a study period. Incidence rates (IRs) were based on the number of events per 100 person-years of exposure, with entire time on treatment considered the exposure time rather than time until the first event. If a patient had multiple events, all events were counted.
3 Results
In both UNCOVER-2 and -3, baseline characteristics of etanercept responders were generally comparable to those of non-responders, except for weight, which was numerically higher in the non-responder (as defined by either sPGA 0/1 or PASI 75) versus responder groups (Table 1).
At Week 12 in UNCOVER-2, 64% of etanercept-treated patients, 27.1% of ixekizumab Q4W-treated patients, and 16.8% of ixekizumab Q2W-treated patients were sPGA 0/1 non-responders. Among the etanercept-treated patients who did not reach sPGA 0/1 at Week 12 and switched to ixekizumab Q4W (n = 200), 73.0% had an sPGA 0/1, 83.5% had a PASI 75, 57.0% had a PASI 90, and 22.0% had a PASI 100 after 12 weeks of treatment with ixekizumab Q4W (Week 28) (Fig. 1a). After 44 weeks (Week 60) of treatment with ixekizumab Q4W, among induction-period sPGA 0/1 non-responders to etanercept, 71.0% achieved an sPGA 0/1, 82.5% achieved a PASI 75, 68.5% achieved a PASI 90, and 43.5% achieved a PASI 100 (Fig. 1a; Table 2). sPGA 0/1, PASI 75, 90, and 100 responses for placebo-treated patients who were sPGA 0/1 non-responders at Week 12 and treated with ixekizumab from Weeks 12–60 (48 weeks of treatment with ixekizumab Q4W) are presented in Fig. 1b and Table 2. At Week 60, the percent improvement in PASI and percentages of patients achieving nail psoriasis area and severity index (NAPSI) and psoriasis scalp severity index (PSSI) scores of 0 were also similarly high in non-responders to both placebo and etanercept after switching to ixekizumab (Table 2).
UNCOVER-2: sPGA and PASI response rates through Week 60 in Week 12 sPGA 0/1 non-responders. sPGA 0/1, PASI 75, PASI 90, and PASI 100 response rates among patients in UNCOVER-2 who were switched to ixekizumab Q4W if they were Week 12 sPGA 0/1 non-responders to etanercept (a) or placebo (b). Response rates for all patients treated with etanercept (a) or placebo (b) during the first 12 weeks have been previously reported and are provided for reference [1]. ETN etanercept, ETN-NR/IXE Q4W etanercept Weeks 0–12, placebo at Week 12, and 80 mg ixekizumab every 4 weeks for Weeks 16–60, NR sPGA 0/1 non-responder at Week 12, PASI psoriasis area and severity index, PBO placebo, PBO-NR/IXE Q4W placebo Weeks 0-12 and 80 mg ixekizumab every 4 weeks for Weeks 16–60 after a starting dose of 160 mg at Week 12, sPGA static physician global assessment
At Week 12 in UNCOVER-3, 46.6% of etanercept-treated patients, 15.8% of ixekizumab Q4W-treated patients, and 12.7% of ixekizumab Q2W-treated patients were PASI 75 non-responders. Among the etanercept-treated patients who were PASI 75 non-responders at Week 12 and switched to ixekizumab Q4W (n = 165), 78.2% had a PASI 75, 58.8% had a PASI 90, and 27.3% had a PASI 100 after 12 weeks of treatment with ixekizumab Q4W (Week 28) (Fig. 2a). As described previously with UNCOVER-2, long-term efficacy was also observed in UNCOVER-3, with 77.6% of etanercept PASI 75 non-responders achieving a PASI 75, 67.9% achieving a PASI 90, and 43.0% achieving a PASI 100 after 44 weeks (Week 60) of treatment with ixekizumab Q4W (Fig. 2a; Table 2). PASI 75, 90, and 100 responses for placebo-treated patients who were PASI 75 non-responders at Week 12 and treated with ixekizumab from Weeks 12 to 60 (48 weeks of treatment with ixekizumab Q4W) are also presented in Fig. 2b and Table 2. As in UNCOVER-2, in UNCOVER-3 the percent improvement in PASI, and percentages of patients achieving NAPSI and PSSI scores of 0 for patients who switched to ixekizumab after non-response to etanercept or placebo were similarly high at Week 60 (Table 2).
UNCOVER-3: sPGA and PASI response rates through Week 60 in Week 12 PASI 75 non-responders. sPGA 0/1, PASI 75, PASI 90, and PASI 100 response rates among patients in UNCOVER-3 who were switched to ixekizumab Q4W if they were Week 12 PASI 75 non-responders to etanercept (a) or placebo (b). Response rates for all patients treated with etanercept (a) or placebo (b) during the first 12 weeks have been previously reported and are provided for reference [1]. ETN etanercept, ETN-NR/IXEQ4W etanercept Weeks 0–12, placebo at Week 12, and 80 mg ixekizumab every 4 weeks for Weeks 16–60, NR PASI 75 non-responder at Week 12, PASI psoriasis area and severity index, PBO placebo, PBO-NR/IXEQ4W placebo Weeks 0-12 and 80 mg ixekizumab every 4 weeks for Weeks 16–60 after a starting dose of 160 mg at Week 12, sPGA static physician global assessment
Overall, safety profiles, represented as incidence rates of treatment-emergent adverse events (TEAEs) of any severity, serious adverse events (AEs), or AEs leading to discontinuation in the two subgroups of non-responders to placebo or etanercept in UNCOVER-2 or -3 were generally comparable after switching to ixekizumab (Table 3). Furthermore, there were also no outstanding differences in incidence rates of adverse events of special interest, including injection-site reactions, infections, Candida infections, and inflammatory bowel disease in either subgroup in either trial after switching to ixekizumab (Table 3).
4 Discussion
In both trials, high percentages of patients who were sPGA 0/1 (UNCOVER-2) or PASI 75 (UNCOVER-3) etanercept non-responders during the induction period achieved sPGA 0/1, PASI 75, 90, and 100 after 12 weeks and maintained this response through 44 weeks following the switch to ixekizumab Q4W, demonstrating a potential long-term benefit in switching to ixekizumab following non-response to etanercept. Responses to etanercept did not appear to be impacted substantially by differences in baseline characteristics, with the potential exception of weight. This is generally consistent with previously established findings, where greater percentages of patients in lower weight (or body mass index [BMI]) categories had higher responses than patients in higher weight (or BMI) categories, although weight category cut points varied by study [7, 8].
There is currently variability in guidance regarding what defines a non-response that warrants change in treatment. sPGA 0/1 or PASI 75 has frequently been the treatment goal in phase III clinical trials; however, non-response has often been defined as failure to achieve PASI 50. Furthermore, current guidelines by the European Consensus Program [9] recommend remaining on the same treatment regimen unless the following conditions apply: reduction in PASI of <50%, or a reduction in PASI of at least 50% and <75% combined with a DLQI (Dermatology Life Quality Index) score >5. The results of the present analysis suggest that PASI 75 or sPGA 0/1 non-responders may indeed benefit from switching, although neither of these trials had a comparator group that continued receiving etanercept to determine the full level of benefit from switching over time.
Of note, it has been recommended that clinicians should wait four times the terminal biologic half-life to ensure that the first biologic has been cleared from the body before starting a second biologic [10]. Interestingly, a recent consensus report from the Transitioning Therapies program, created by dermatologists from 33 countries, suggests using a washout period when patients are switching biologic therapies for safety concerns but not when switching due to lack of efficacy [11]. In UNCOVER-2 and -3, five times the terminal half-life was deemed appropriate for a drug under development. Hence, the response to ixekizumab without a washout in etanercept-treated patients was not assessed.
There are several limitations to these analyses. These studies were not designed to directly compare outcomes in etanercept non-responders versus placebo non-responders switched to ixekizumab Q4W. Additionally, while patients and investigators in UNCOVER-2 remained blinded through Week 60, patients and investigators in UNCOVER-3 were not blinded after the first 12 weeks of treatment, potentially resulting in higher responses after Week 12 in that trial. Another potential limitation is that some patients may require more than 12 weeks to achieve sPGA 0/1 or PASI 75 in response to etanercept; therefore, it is conceivable that with longer exposure to etanercept, some of the non-responders might have become responders. The ixekizumab dosing regimen currently approved in several markets for the treatment of moderate-to-severe plaque psoriasis includes a 160-mg starting dose at Week 0 followed by 12 weeks of 80 mg ixekizumab every 2 weeks [9]. It is possible that responses following the switch to ixekizumab may have been higher if this induction dosing regimen had been used in the etanercept non-responders at Week 16.
Like the results found in the present analyses, the ACCEPT study showed that many patients who were non-responders to etanercept experienced a PASI 75 response after switching to ustekinumab [5]. However, in contrast to the ACCEPT study, in both UNCOVER-2 and -3, patients who switched to ixekizumab Q4W after non-response to etanercept had similarly high responses (84 and 78% had a PASI 75; 57% and 59% had a PASI 90) as patients treated with ixekizumab Q4W as induction therapy (78 and 84% had a PASI 75; 60 and 65% had a PASI 90) following 12 weeks of treatment [1, 5]. This supports the notion that switching an etanercept non-responder to a biologic that inhibits IL-17A may be a clinically beneficial strategy. Of note, there are currently no guideline recommendations regarding optimal sequence of therapies for patients with psoriasis and whether patients would benefit from a faster/higher level of clearance at early stages of treatment.
5 Conclusions
Patients with moderate-to-severe plaque psoriasis who were non-responders to etanercept therapy after 12 weeks, as defined by different criteria (i.e., sPGA 0/1 and PASI 75), had high levels of clinical responses after being switched to 80 mg ixekizumab Q4W. This response was attained quickly and maintained through Week 60 in two phase III trials, with similar safety profiles for both placebo non-responders and etanercept non-responders who switched to ixekizumab.
Change history
29 March 2018
The article Efficacy and Safety of Switching to Ixekizumab in Etanercept Non-Responders: A Subanalysis from Two Phase III Randomized Clinical Trials in Moderate-to-Severe Plaque Psoriasis (UNCOVER-2 and -3) written by Andrew Blauvelt
References
Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51.
Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–56.
Fellner C. More Psoriasis Biologic therapies expected to treat advanced plaque. P T. 2016;41(6):388–90.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–28.
Clemmensen A, Spon M, Skov L, Zachariae C, Gniadecki R. Responses to ustekinumab in the anti-TNF agent-naive vs. anti-TNF agent-exposed patients with psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2011;25(9):1037–40.
Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58(3):443–6.
Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25(9):1007–11.
Mrowietz U. Implementing treatment goals for successful long-term management of psoriasis. J Eur Acad Dermatol Venereol. 2012;26(Suppl 2):12–20.
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161(5):987–1019.
Mrowietz U, de Jong EM, Kragballe K, Langley R, Nast A, Puig L, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28(4):438–53.
Acknowledgements
The authors thank Bridget Charbonneau for her writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
NCT01597245 and NCT01646177 and the post hoc analyses of these studies presented in this manuscript were funded by Eli Lilly and Company.
Conflict of interest
Andrew Blauvelt served as a scientific advisor and clinical study investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Genentech, GSK, Janssen, Eli Lilly and Company, Merck, Novartis, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun, UCB, and Valeant, and is a paid speaker for Eli Lilly and Company. Kim A. Papp has served as a consultant for 3 M, Abbott/AbbVie, Akesis, Akros, Alza, Amgen, Astellas, Baxter, Bayer, Boehringer Ingelheim, Celgene, Cipher, Coherus, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Funxional Therapeutics, Galderma, Genentech, Janssen, J&J, Kyowa Hakko Kirin, Leo, Lypanosys, Medimmune, Meiji Seika Pharma Co., Merck, Mitsubishi Pharma, Mylan, Novartis, Pan GEnetics, Pfizer, Regeneron Pharmaceuticals, Merck-Serono, Stiefel, Sosei, Takeda, UCB, Vertex, Wyeth, and Xoma; he has served as part of a speakers bureau for 3 M, Abbott, AbbVie, Amgen, Astellas, Celgene, Genentech, Merck, Novartis, Pfizer, and Merck-Serono; he has received clinical research grants from Abbott/AbbVie, Allergan, Amgen, Anacor, Astellas, Boehringer Ingelheim, Celgene, Celtic, Cipher, Coherus, Dermira, Dow Pharma, Eli Lilly, Galderma, GSK, Janssen, Kyowa Hakko Kirin, Leo, Medimmune, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Merck-Serono, Stiefel, Takeda, UCB, Wyeth, and Xoma; he has received honoraria from Abbott/AbbVie, Akros, Amgen, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Forward Pharma, Galderma, Janssen, Kyowa Hakko Kirin, Lypanosys, Meiji Seika Pharma, Merck, Mitsubishi Pharma, Mylan, Novartis, Pfizer, Merck-Serono, Stiefel, Takeda, UCB, and Vertex; he has served as a scientific officer for Abbott/AbbVie and Anacor; he has served on a scientific committee for Celgene, Centocor, Janssen, Novartis, Pfizer, and UCB; he has served on advisory boards for 3 M, Abbott/AbbVie, Allergan, Alza, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen, Janssen Biotech (Centocor), Leo, Medimmune, Merck, Mylan, Novartis, Pfizer, Regeneron Pharmaceuticals, Merck-Serono, UCB; and he has served as a coordinating investigator for Astellas, Boehringer Ingelheim, Celgene, Coherus, Dermira, Eli Lilly, Forward Pharma, Isotechnika, Kyowa hakko Kirin, Leo, Merck, Novartis, Pfizer, Merck-Serono, Takeda, and Xoma. Christopher E.M. Griffiths has received research grants and/or has received honoraria from AbbVie, BMS, GSK, Janssen, Leo Pharma, MSD, Pfizer, Novartis, Sandoz, Eli Lilly and Company, and UCB Pharma. Lluis Puig has been a clinical trial investigator for AbbVie, Amgen, GSK, Janssen, Eli Lilly and Company, MSD, Novartis, Pfizer, Regeneron, and VBL; he has also been a paid advisor/speaker for AbbVie, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, GSK, Janssen, Leo-Pharma, Eli Lilly and Company, Merck-Serono, MSD, Novartis, Pfizer, Regeneron, Sandoz, Sanofi, and VBL. Jamie Weisman has served as a clinical study investigator for Amgen, AbbVie, Biogen, Boehringer Ingelheim, Celgene, Glaxo Smith Kline, Janssen, Leo pharmaceuticals, Eli Lilly and Company, Merck, Novartis, Pfizer, Regeneron, Stiefel, and Valeant; she has also served on speaker’s bureaus/advisory boards for AbbVie, Janssen, and Eli Lilly and Company. Yves Dutronc, Lisa Farmer Kerr, Dapo Ilo, and Lotus Mallbris are stockholders and full-time employees of and Eli Lilly and Company. Matthias Augustin has been a scientific consultant for Leo Pharma, Almirall, Abbott Laboratories, Amgen, Biogen Idec, Celgene, Centocor Ortho Biotech, Janssen, Medac Pharma, Merck, Novartis, and Pfizer; he has been a clinical study investigator for Eli Lilly and Company, Leo Pharma, Abbott Laboratories, Amgen, Biogen Idec, Celgene, Centocor Ortho Biotech, Janssen, Merck, Novartis, and Pfizer; and he has received honoraria from Eli Lilly and Company, Leo Pharma, Abbott Laboratories, Amgen, Biogen Idec, Celgene, Centocor Ortho Biotech, Janssen, Almirall, Medac Pharma, Merck, Novartis, and Pfizer.
Ethics Approval and Consent to Participate
This report contains post hoc analyses of previously published studies (Griffiths CE, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-51 and Gordon KB, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-56). Study protocols and informed consent forms for NCT01597245 and NCT01646177 were approved by an investigational review board at each site, all patients signed informed consent before undergoing study-related procedures, and the trials were conducted in accordance with the ethical principles of the Declaration of Helsinki.
Rights and permissions
Open Access The article is forthwith distributed under the terms of the Creative Commons Attribution Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, a link is provided to the Creative Commons license and any changes made are indicated.
About this article
Cite this article
Blauvelt, A., Papp, K.A., Griffiths, C.E.M. et al. Efficacy and Safety of Switching to Ixekizumab in Etanercept Non-Responders: A Subanalysis from Two Phase III Randomized Clinical Trials in Moderate-to-Severe Plaque Psoriasis (UNCOVER-2 and -3). Am J Clin Dermatol 18, 273–280 (2017). https://doi.org/10.1007/s40257-016-0246-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-016-0246-9