Abstract
Introduction
Gaucher disease (GD) is an inherited recessive enzyme deficiency with a multisystem condition. The Iranian government covers the therapeutic expenditure of GD patients as it is not affordable for the patients. The aim of this study is to identify the main components of the cost of care in Gaucher patients (GPs) in Iran.
Methods
The Gaucher patients were identified from the Iran Food and Drug Administration (IFDA) national registry database. The direct medical costs, including medication, diagnostic services, and physician visits were considered. The prices of therapeutic and diagnostic services were extracted from Iranian medical tariff book 2014–15. Iran Food and Drug Administration determined the cost of medications.
Results
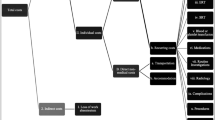
164 Gaucher patients have been registered in Iran. A valid and reliable diagnostic tests are not used to identify the type of GD. The average health care cost per annum was 20,758 USD per patient, which is higher than 4 GDP per capita in Iran. Medication cost constitutes 95.2% of the total cost. The average cost of each GP was $1,473,818 in his/her total life.
Conclusion
GD is amongst the high-cost diseases and should be managed effectively. The application of oral medication for eligible GPs could improve allocative efficiency in GD management significantly. A sound, valid and reliable national clinical guideline could improve the efficiency of healthcare resources effectively. Selecting appropriate strategies for reducing the birth of a child with Gaucher, could support allocative efficiency of the limited resources effectively.

Similar content being viewed by others
References
Biegstraaten M, Van Schaik I, Aerts J, Hollak C. Non-neuronopathic’Gaucher disease reconsidered. Prevalence of neurological manifestations in a Dutch cohort of type I Gaucher disease patients and a systematic review of the literature. J Inherit Metab Dis. 2008;31(3):337–49.
Keatinge M, Bui H, Menke A, Chen Y-C, Sokol AM, Bai Q, et al. Glucocerebrosidase 1 deficient Danio rerio mirror key pathological aspects of human Gaucher disease and provide evidence of early microglial activation preceding alpha-synuclein-independent neuronal cell death. Hum Mol Genet. 2015;24(23):6640–52.
Machaczka M. What hematologist needs to know about Gaucher disease. Acta Haematol Pol. 2013;44:301–6.
Zimran A. How I treat Gaucher disease. Blood. 2011;118(6):1463–71.
Mistry P, Weinthal J, Weinreb N. Disease state awareness in Gaucher disease: a Q&A expert roundtable discussion. Clin Adv Hematol Oncol: H&O. 2012;10(6 Suppl 8):1–16.
Barranger JA, Brady RO, Grabowski GA, Mankin H, Mistry PK, Weinreb NJ. Position statement: National Gaucher Foundation Medical Advisory Board, January 7, 2014. Am J Hematol. 2014;89(5):457–8.
Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement therapy for inherited enzyme deficiency—macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324(21):1464–70.
Gonzalez DE, Turkia HB, Lukina EA, Kisinovsky I, Dridi MFB, Elstein D, et al. Enzyme replacement therapy with velaglucerase alfa in Gaucher disease: results from a randomized, double-blind, multinational, phase 3 study. Am J Hematol. 2013;88(3):166–71.
Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122(1):33–9.
Weinreb NJ, Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher registry. Am J Med. 2002;113(2):112–9.
Charrow J, Dulisse B, Grabowski G, Weinreb N. The effect of enzyme replacement therapy on bone crisis and bone pain in patients with type 1 Gaucher disease. Clin Genet. 2007;71(3):205–11.
Wenstrup R, Roca-Espiau M, Weinreb N, Bembi B. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75(suppl_1):A2–A12.
Elstein D, Hadas-Halpern I, Itzchaki M, Lahad A, Abrahamov A. Effect of low-dose enzyme replacement therapy on bones in Gaucher disease patients with severe skeletal involvement. Blood Cell Mol Dis. 1996;22(2):104–11.
Chen M, Wang J. Gaucher disease: review of the literature. Arch Pathol Lab Med. 2008;132(5):851–3.
Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, et al. Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385(9985):2355–62.
Mignot C, Doummar D, Maire I, De Villemeur TB, Group FTGDS. Type 2 Gaucher disease: 15 new cases and review of the literature. Brain Dev. 2006;28(1):39–48.
Weinreb NJ, Aggio MC, Andersson HC, Andria G, Charrow J, Clarke JT, et al., editors. Gaucher disease type 1: revised recommendations on evaluations and monitoring for adult patients. Seminars in hematology; 2004: Elsevier.
Weinreb NJ, Deegan P, Kacena KA, Mistry P, Pastores GM, Velentgas P, et al. Life expectancy in Gaucher disease type 1. Am J Hematol. 2008;83(12):896–900.
World Health Organization (WHO). Global Health Observatory (GHO) date 2015. Available from: http://search.who.int/search?q=iran+life+expectancy&ie=utf8&site=who&client=_en_r&proxystylesheet=_en_r&output=xml_no_dtd&oe=utf8&getfields=doctype. Accessed 2 Dec 2017.
Belmatoug N, Di Rocco M, Fraga C, Giraldo P, Hughes D, Lukina E, et al. Management and monitoring recommendations for the use of eliglustat in adults with type 1 Gaucher disease in Europe. Eur J Intern Med. 2017;37:25–32.
Smid BE, Hollak CE. A systematic review on effectiveness and safety of eliglustat for type 1 Gaucher disease. Expert Opin Orphan Drugs. 2014;2(5):523–9.
Krug B, Schwartz I, De Oliveira FL, Alegra T, Martins NC, Todeschini L, et al. The management of Gaucher disease in developing countries: a successful experience in Southern Brazil. Public Health Genomics. 2010;13(1):27–33.
Acknowledgements
We would like to acknowledge the IFDA staffs, particularly Dr. Zolfaghar Taghaviyan who were supportive during data collection from IFDA database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Faculty of pharmacy and Research Ethics Committee of the Tehran University of Medical Sciences.
Conflicts of interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davari, M., Nabizadeh, A., Kadivar, M. et al. Healthcare resource utilization and cost of care for Gaucher patients in Iran. J Diabetes Metab Disord 18, 127–132 (2019). https://doi.org/10.1007/s40200-019-00399-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-019-00399-x