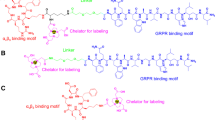
Abstract
Background
Integrins are interesting targets in oncology. RGD sequence has high affinity for αVβ3 integrin receptors. Diagnostic/therapeutic agents can be selectively delivered into cancer cells overexpressing αVβ3 integrin by using RGD as a carrier. Nonsteroidal anti-inflammatory drugs (NSAIDs) have shown anticancer properties in in vitro and in vivo studies. The anti-cancer properties of NSAIDs occur though COX-2 inhibition. Regarding the anti-cancer properties of NSAIDs and overexpression of COX-2 enzyme in cancer cells, targeted delivery of NSAIDs into cancer cells to maximize their efficiency and minimize their side effects may gain increased clinical interest.
Objectives
In this study, RGD was conjugated to ketoprofen/Naproxen to selectively transfer these non-selective COX inhibitors into cancer cells.
Methods
Keto/Nap-RGD-N4 peptides were synthesized based on solid phase fmoc peptide synthesis. Radiolabeling with [99mTc] via N4 (GGAG) ligand was done for biological evaluation. Affinity and specificity of Keto/Nap-RGD-N4 to integrin was determined using A2780, OVCAR-3, SKOV-3 and HT-1080 cell lines. Percentage of Intenalization was measured in A2780 cells. Biodistriburion was studied in normal and tumor model mice.
Results
Radiolabeled compounds showed high affinity to cells expressing αVβ3 integrin in comparison to cells not expressing αVβ3. The affinity to A2780 was significantly higher than OVCAR-3 cells. The %internalization into A2780 cells was quite low. Compounds showed more than 50% inhibition on A2780 and OVCAR-3 cells, less than 10% on MCF-7 and HT-1080 cells and no cytotoxicity on fibroblast cells after 48 h incubation. Although uptake of radiolabeled compounds in tumor was high at 1 h post-injection, the tumor/blood ratio was less than 1.5 which made SPECT imaging impossible.
Conclusion
Provided that NSAID drugs are conjugated to RGD, there will be a selective delivery to target tissues as well as synergetic anti-tumor effects which reduce systemic doses and toxicity.

Graphical abstract






Similar content being viewed by others
References
Soudy R, Chen C, Kaur K. Novel peptide–Doxorubucin conjugates for targeting breast Cancer cells including the multidrug resistant cells. J Med Chem. 2013;56(19):7564–73.
Dal Pozzo A, Ni M-H, Esposito E, Dallavalle S, Musso L, Bargiotti A, et al. Novel tumor-targeted RGD peptide–Camptothecin conjugates: synthesis and biological evaluation. Bioorg Med Chem. 2010;18(1):64–72.
Chen X, Plasencia C, Hou Y, Neamati N. Synthesis and Biological Evaluation of Dimeric RGD Peptide−Paclitaxel Conjugate as a Model for Integrin-Targeted Drug Delivery. J Med Chem. 2005;48(4):1098–106.
Le Joncour V, Laakkonen P. Seek & Destroy , Use of Targeting Peptides for Cancer Detection and Drug Delivery. Bioorg Med Chem. 2018;26(10):2797–806.
Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and Peptidomimetics for designing selective inhibitors of Theαvβ3 integrin for a new Cancer therapy. Angew Chem Int Ed Eng. 1997;36(1314):1374–89.
Rüegg, C.; Dormond, O.; Mariotti, A. Endothelial Cell Integrins and COX-2: Mediators and Therapeutic Targets of Tumor Angiogenesis. Biochimica et Biophysica Acta - Reviews on Cancer. 2004; pp 51–67.
Danhier F, Le Breton A, Préat V. RGD-based strategies to target alpha(v) Beta(3) integrin in Cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961–73.
Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, et al. A Pilot Trial of Vitaxin, A Humanized Anti-Vitronectin Receptor (Anti αvβ3 ) Antibody in Patients with Metastatic Cancer. Cancer Biother Radiopharm. 2001;16(2):125–32.
Gaertner FC, Kessler H, Wester H-J, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(S1):126–38.
Jin S, Wang Y, Zhu H, Wang Y, Zhao S, Zhao M, et al. Nanosized Aspirin-Arg-Gly-Asp-Val: delivery of aspirin to Thrombus by the target carrier Arg-Gly-Asp-Val Tetrapeptide. ACS Nano. 2013;7(9):7664–73.
Colombo R, Mingozzi M, Belvisi L, Arosio D, Piarulli U, Carenini N, et al. Synthesis and biological evaluation (in vitro and in vivo) of cyclic arginine–Glycine–aspartate (RGD) Peptidomimetic–paclitaxel conjugates targeting integrin α V β 3. J Med Chem. 2012;55(23):10460–74.
Dormond O, Foletti A, Paroz C, Rüegg C. NSAIDs inhibit ΑVβ3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat Med. 2001;7(9):1041–7.
Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for Cancer therapy. Mol Cell Pharmacol. 2009;1(1):29–43.
Shiff SJ, Shivaprasad P, Santini DL. Cyclooxygenase inhibitors: drugs for Cancer prevention. Curr Opin Pharmacol. 2003;3(4):352–61.
Liu S, Edwards DS. 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev. 1999;99(9):2235–68.
Yapp DTT, Ferreira CL, Gill RK, Boros E, Wong MQ, Mandel D, et al. Imaging tumor vasculature noninvasively with positron emission tomography and RGD peptides labeled with copper 64 using the Bifunctonal chelates DOTA, Oxo-DO3a. and PCTA. Mol. Imaging. 2013;12(4):263–72.
Yoshimoto M, Ogawa K, Washiyama K, Shikano N, Mori H, Amano R, et al. α v β 3 integrin-targeting radionuclide therapy and imaging with monomeric RGD peptide. Int J Cancer. 2008;123(3):709–15.
Dijkgraaf I, Yim C-B, Franssen GM, Schuit RC, Luurtsema G, Liu S, et al. PET imaging of Αvβ3 integrin expression in Tumours with 68Ga-Labelled Mono-, Di- and Tetrameric RGD peptides. Eur J Nucl Med Mol Imaging. 2011;38(1):128–37.
Tsiapa I, Loudos G, Varvarigou A, Fragogeorgi E, Psimadas D, Tsotakos T, et al. Biological evaluation of an ornithine-modified 99mTc-labeled RGD peptide as an angiogenesis imaging agent. Nucl Med Biol. 2013;40(2):262–72.
Stewart, John Morrow, Young, J. D. Solid Phase Peptide Synthesis; 1984.
Rezaeianpour S, Bozorgi AH, Moghimi A, Almasi A, Balalaie S, Ramezanpour S, et al. Synthesis and biological evaluation of cyclic [99mTc]-HYNIC-CGPRPPC as a fibrin-binding peptide for molecular imaging of thrombosis and its comparison with [99mTc]-HYNIC-GPRPP. Mol Imaging Biol. 2017;19(2):256–64.
Khoshbakht S, Tabib K, Soraya Shahhosseini B, Shahhosseini S, Kobarfard F, Beiki D, et al. HYNIC a Bifunctional prosthetic Group for the Labelling of peptides with 99m Tc and 18 FDG. J Radioanal Nucl Chem. 2016;307:1125–34.
Khoshbakht S, Beiki D, Geramifar P, Kobarfard F, Sabzevari O, Amini M, et al. 18FDG-Labeled LIKKPF: A PET Tracer for Apoptosis Imaging. J Radioanal Nucl Chem. 2016;310(1).
Shokri B, Zarghi A, Shahhoseini S, Mohammadi R, Kobarfard F. Design, synthesis and biological evaluation of Ketoprofen conjugated to RGD/NGR for targeted Cancer therapy. Iran J Pharm Res IJPR. 2018;17(4):1297–305.
Zarghi A, Ghodsi R. Design, synthesis, and biological evaluation of Ketoprofen analogs as potent Cyclooxygenase-2 inhibitors. Bioorg Med Chem. 2010;18(16):5855–60.
Shokri B, Zarghi A, Shahhoseini S, Mohammadi R, Kobarfard F. Design, Synthesis and Biological Evaluation of Peptide-NSAID Conjugates for Targeted Cancer Therapy. Arch Pharm (Weinheim). 2019;352(8):1800379.
Liu S. Radiolabeled Multimeric cyclic RGD peptides as integrin α v β 3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3(5):472–87.
Knetsch PA, Zhai C, Rangger C, Blatzer M, Haas H, Kaeopookum P, et al. [(68)Ga]FSC-(RGD)3 a Trimeric RGD peptide for imaging Αvβ3 integrin expression based on a novel Siderophore derived chelating scaffold-synthesis and evaluation. Nucl Med Biol. 2015;42(2):115–22.
Zhao Z-Q, Yang Y, Fang W, Liu S. Comparison of biological properties of 99mTc-labeled cyclic RGD peptide Trimer and dimer useful as SPECT radiotracers for tumor imaging. Nucl Med Biol. 2016;43(11):661–9.
Acknowledgments
This research was supported by Shahid Beheshti University of Medical Sciences, Tehan, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi, R., Shokri, B., Shamshirian, D. et al. Synthesis and biological evaluation of RGD conjugated with Ketoprofen/Naproxen and radiolabeled with [99mTc] via N4(GGAG) for αVβ3 integrin-targeted drug delivery. DARU J Pharm Sci 28, 87–96 (2020). https://doi.org/10.1007/s40199-019-00318-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00318-8