Abstract
Drinking water treatment residuals (WTRs) are wastes generated when water is clarified using aluminium or iron salts. They are increasingly being considered as a resource with potential reuse value, particularly in relation to soil or water remediation. Adsorption–desorption capacity of Al-based (Al-WTR) and Fe-based (Fe-WTR) materials was investigated here for Pb and Zn, both separately and in combination, as a preliminary trial to assess their utility for immobilising contaminant metals in environmental settings. Maximum adsorption observed at the highest test solution concentrations imposed (400 mg/L) was similar for each WTR type and each metal; Al-WTRs sorbed Zn at 3579 mg/kg and Pb at 4025 mg/kg, while Fe-WTRs sorbed Zn and Pb at 3579 mg/kg and 3980 mg/kg, respectively. Equilibrium adsorption data were tested against Langmuir, Freundlich, and Temkin isotherm models, which indicated a substantial reserve capacity for further Pb sorption and that multiple sorption mechanisms were involved. Subsequent desorption tests with 0.001 M CaCl2 solution indicated that > 89.76% of sorbed metal remained sorbed. When in solution together, both metals were strongly sorbed by WTRs, but a slight preference for Pb was observed. The results indicate that WTRs would be very effective immobilising agents if placed in contaminated soil or if used to treat contaminated waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of raw water for drinking typically involves the addition of aluminium (Al) or iron (Fe) salts as flocculants and coagulants during the water treatment process, which results in the generation of a sludge-like by-product containing the flocculants and the removed impurities that are often referred to as water treatment residuals (WTRs) (Howe et al. 2012).
Vast quantities of WTRs (i.e. in the millions to tens of millions of tons) are generated annually across the globe (Basibuyuk and Kalat 2004), with varying proportions of those produced from application of aluminium-based salts (generating what can be referred to as Al-WTR) and those from ferric-based salts (Fe-WTR). One estimate, for the USA alone, was that more than 2 million tons of WTRs are generated every day (Prakash and SenGupta 2003). WTRs may be disposed of in various ways, including burial in general landfill in jurisdictions such as the European Union within which they are classified as inert waste under EU Council Directive 99/31/EC (1999; as cited in Keeley et al. 2014). Substantial amounts of WTRs are indeed sent to landfills for disposal in many countries, and the economic expense of this disposal route is continually rising. However, in addition to the growing expense, landfill disposal of WTRs is increasingly recognised as wasting a potentially useful resource, with reuse options including in water and wastewater treatment, soil improvement and construction (Babatunde and Zhao 2007; Ippolito et al. 2011; Turner et al. 2019). Beneficial use of WTRs is therefore increasingly favoured, offering financial advantages and helping to develop a more circular economy with greater levels of materials recycling. However, to determine the ultimate utility of WTRs for removing contaminants from wastewater and/or for immobilising contaminants in soil and sediment pore water, the sorption and retention capacity of WTRs for contaminants must first be established and understood. Such knowledge is also important in terms of placing the use of these and other recovered wastes for pollution management in the wider context of pollution control options now available, including those offered by recently chemically engineered sorbent materials (Liu 2021).
The aims of the study, therefore, were to investigate adsorption and retention by two types of WTRs (one generated at a plant using Al salts, Al-WTRs, and the other generated from the use of Fe salts, Fe-WTRs) of two important potentially toxic elements, Pb and Zn, that are highly prevalent contaminants in waters and wastes and are on the priority substances (in the case of Pb) and specific pollutants (in the case of Zn) lists under the Water Framework Directive of the European Union and on the Priority Pollutant List under the Clean Water Act of the USA.
Adsorption and retention of Pb and Zn were tested both singularly and in combination, the latter in order to assess the effects of cation adsorption competition. Adsorption isotherms (Henry’s, Langmuir, Freundlich and Temkin) were fitted to the data in order to describe, model and examine the adsorption processes.
Materials and methods
Water treatment residuals source and preparation
Water treatment residuals (WTRs) from two water treatment plants in Staffordshire, England, were provided by Severn Trent Water. One plant primarily uses Al salts and the other Fe-based salts, generating what is designated here as Al-WTRs and Fe-WTRs, respectively. According to the results from a previous project carried out on the same materials (Howells et al. 2018), the properties of Al-WTRs once dried were pH 7.34 ± 0.06, Al content 11.64 ± 1.08% w/w, organic matter (OM) content 28.0 ± 0.1% w/w and Fe content 0.91 ± 0.08% w/w, while those of Fe-WTRs were pH 7.37 ± 0.01, Fe 17.69 ± 0.19% w/w, OM 25.9 ± 0.2% w/w and Al 0.71 ± 0.12% w/w. For this study, the as-received (i.e. moist) WTRs were dried at 30 °C until they became a stable mass and were ground to pass a 2-mm sieve.
Water treatment residuals (WTRs) adsorption capacity for lead and zinc
Following commonly employed batch exchange procedures for determining the adsorption capacity of a substance, 2.0 g WTR samples were equilibrated with 20 mL solutions with Pb or Zn concentrations of 10, 50, 100, 200, 300 or 400 mg/L (3 replicates for each WTR type at each concentration). Samples were equilibrated via end-over-end shaking (40 rpm; ~ 20 °C) for 24 h, which is generally recognised as sufficient time to establish equilibrium (e.g. Coles and Yong 2006). Equilibration was immediately followed by centrifugation, filtration of supernatant using 0.45-µm syringe filters, acidification with a drop of trace analysis grade concentrated nitric acid (Primar Plus) and then analysis for metals using ICP-OES (Varian vista) (e.g. as per Dada et al. 2012). Concentrations determined in the equilibrium solutions were used to calculate amounts of metal sorbed to WTRs, and the data were further examined through constructing Henry’s, Langmuir, Freundlich and Temkin adsorption isotherms models, as outlined in Table 1. Equation 1, which was reported by Vanderborght and Van Grieken (1977), is used to calculate the quantity of sorbate retained by a unit of mass of the sorbent Qeads (mg/g).
where C0 = initial solution concentration before adsorption; Ceads = concentration at adsorption equilibrium; V = volume of the adsorbate, and m = mass of WTRs in grams.
The adsorbed amount of Zn or Pb by each of Al-WTR and Fe-WTR can be expressed as an adsorption percentage (Eq. 2), which is based on the ratio of the mass of adsorbed ions at equilibrium to the initial mass of adsorbate ions in solution (OECD 2000).
At its simplest, adsorption of a metal (or other substance) from solution into solid can be expressed as Henry’s adsorption isotherm, with KH typically denoted as Henry’s adsorption constant (units of L/g). However, adsorption onto a solid across a wide concentration range is not typically constant. In order to understand some of the adsorption characteristics of Zn and Pb into WTRs, the experimental data were fitted to Henry’s, Langmuir, Freundlich and Temkin linearised equations, all of which have been described in detail elsewhere (Dada et al. 2012; Sparks 2003; Yildirim 2006). From a fitted Langmuir isotherm, one can calculate the Qm value, which refers to the maximum monolayer coverage capacity (mg/g) onto the adsorbent, assuming that the binding energy of the sites is homogenous and can also determine the ‘b’ value which is the term used for the Langmuir isotherm constant (L/mg) (Dada et al. 2012; Bonilla-Petriciolet et al. 2017).
According to the Temkin isotherm, the heat of the adsorption for all adsorbates decreases linearly as the amount of the adsorbed materials on the sorbent increases. An important calculated parameter for this isotherm is often designated the ‘B’ value, which is the constant related to heat of sorption (J/mol); the AT value is the Temkin isotherm equilibrium constant (L/g) (Dada et al. 2012; Bonilla-Petriciolet et al. 2017). For adsorptions onto adsorbents for which the distribution of heat of adsorption of binding sites is not or cannot be assumed to be uniform, the Freundlich isotherm model is often used. It is a widely applied isotherm for describing adsorption characteristics and generates the following parameters and constants: 1/n, which is dimensionless and is a function of the strength of the adsorption; and Kf is the Freundlich isotherm constant (with units of mg 1−(1/n).g−1.L(1/n)) (Dada et al. 2012; Bonilla-Petriciolet et al. 2017). Although widely used for the purpose, determining characteristics of adsorption processes based on the comparison of Freundlich Kf values can be problematic when 1/n values are not the same or Ceads ≠ 1 because the units of Kf will be different (Chen et al. 1999). To address this, Chen et al. (1999) proposed a unified adsorption variable (Kuads) to unify the unit of Kfads to be L/g. The Kuads is the slope of the isotherm at any value of Ceads or Qeads and can be calculated over a range of Ceads or Qeads using Kfads and 1/n (using Eq. 3 or Eq. 4). Therefore, Kuads is also calculated in the present study:
Thermodynamic data such as the standard adsorption energy can be obtained from Langmuir and Temkin equation using Eq. 5 (Kim et al. 2004):
Desorption of Pb and Zn
The desorbability of Zn and Pb bound to Al-WTRs and Fe-WTRs was determined in batch desorption experiments to determine the degree of reversibility of adsorption by the materials, that is, to determine how well they retain the pollutant metals after exposure to clean solution. Desorption experiments were conducted on the 2 g samples after removal of the supernatant following the initial equilibration process described above for the adsorption experiment. In order to facilitate accurate desorption measurements, and specifically to allow any metals remaining entrained in the WTRs after solution removal to be fully accounted and adjusted for in the calculations, the mass of each tube had been recorded before adding Pb or Zn solution in the sorption experiment and was recorded again at the end of the batch adsorption equilibrium experiments (i.e. after centrifugation and solution removal). The difference in mass enabled calculation of the remaining volume of solution within each tube, and this, together with the measured concentrations of metals in the removed solution, allowed calculation of the amount of entrained metals in that remaining solution.
For desorption, the removed supernatant was replaced by fresh 0.001 M CaCl2 solution and the samples were shaken (40 rpm; ~ 20 °C) again for 24 h, which was immediately followed by centrifugation, filtration using 0.45-µm syringe filters and then analysis for metals using ICP-OES. Concentrations measured in the 0.001 M CaCl2 desorption supernatant solutions (Cedes), corrected for the calculated amounts of entrained metals remaining from the initial sorption solution, were used to express the desorbed amount of Zn or Pb from each of Al-WTRs and Fe-WTRs as a desorption percentage (Eq. 6; OECD 2000).
Adsorption of Zn-Pb ions in combination
In addition to separate adsorption experiments with Zn and Pb individually, adsorption experiments with both Zn and Pb present in solution were conducted to examine competitive adsorption. The experiments were carried out as described above, including the desorption assessment, but with modified initial solution concentrations of (1) 10 mg/L (both Pb and Zn) and (2) 50 mg/L (both Pb and Zn). Statistical assessments of sorption and desorption were conducted via t tests and Mood’s median tests according to data distribution (normality) types using the Real Statistics Resource Pack software (Release 7.6; www.real-statistics.com).
Results and discussion
Results
Water treatment residuals (WTRs) adsorption capacity for lead and zinc
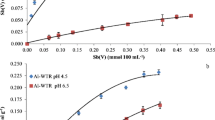
Adsorption of both Pb and Zn was very high at all concentrations tested and on both types of WTRs, with the maximum amounts adsorbed at the highest solution concentrations imposed (400 mg/L) equating to 4025 mg/kg for Pb and 3579 mg/kg for Zn on Al-WTRs (Table 2 and Fig. 1). For Fe-WTRs, it was 3980 mg/kg for Pb and 3579 mg/kg for Zn (Table 2 and Fig. 1). Indeed, in all tested concentrations adsorption was > 92% (thus explaining the very similar maximum adsorption values recorded). Moreover, the realised Zn and Pb adsorption capacities of the Al-WTR and Fe-WTR increased linearly with increasing initial concentration of adsorbate (C0), as shown in Fig. 1, indicating that adsorption maxima had not been reached for either WTR type (Table 2 and Fig. 1).
Adsorption isotherms of Zn and Pb ions in a mono-metal system on Fe-WTR and Al-WTR were created (Fig. 2). The shapes of the isotherms for adsorption of Zn onto both WTRs can be described as being similar to the ‘type I’ or ‘L curve’ isotherm (i.e. asymptotic towards a possible maximum, suggesting that adsorption may be limited to a single monolayer on the surface) (Sparks 2003; Lowell et al. 2012), while Pb adsorption onto Al-WTRs showed a ‘H’ shape or ‘Henry’ isotherm that indicates strong adsorbate–adsorbent interactions such as inner-sphere complex formation (Sparks 2003). The shape of the isotherm for Pb onto Fe-WTRs was intermediate of these, showing aspects of both.
Adsorption isotherms in a mono-metal system for: Al-WTR with Zn, Al-WTR with Pb, Fe-WTR with Zn and Fe-WTR with Pb. Isotherms constructed using Eq. 1 (see main text). Qe is the mass of sorbate (i.e. Pb or Zn) retained per unit mass of the WTR sorbent; Ce is the solution concentration of Pb or Zn at equilibrium. The ICP-OES detection limit for Zn was 0.27 (mg/l) and for Pb was 0.1 (mg/l)
The experimental data reveal that the maximum monolayer coverage capacity (Qm) of the Zn adsorption onto Fe-WTR and Al-WTR from Langmuir isotherm models was 4.38 mg/g and 4.68 mg/g, respectively, and that the Langmuir isotherm constants (b) were 0.13 L/mg for Zn adsorption into both WTRs (Table 3). Adjusted R-squared, R2(adj), values were 0.92 and 0.86 for Zn + Fe-WTRs and Zn + Al-WTRs, respectively, indicating that the adsorption data fitted well to the Langmuir isotherm model. However, the Langmuir isotherm model did not fit Pb adsorption data for either Fe-WTR or Al-WTR (see Table 3), likely reflecting that sorption maxima were not approached for Pb.
From Freundlich isotherms, the 1/n values of Zn adsorption onto Fe-WTR and Al-WTR were 0.68 and 0.74, respectively (Table 3), while 1/n value for Pb adsorption onto Fe-WTR was 0.87. The Freundlich model did not fit the equilibrium adsorption data for Pb onto Al-WTRs. The values of R2(adj) indicate that the Freundlich isotherm did describe the majority of the data accurately for Zn adsorption onto both WTRs, having R2(adj) values of 0.86–0.87 (Table 3), while for Pb adsorption onto Fe-WTR it was 0.56. In order to facilitate better comparison of the Kf values when the 1/n values are different, Ku values were calculated over a range of Ce values (Eq. 3) to unify the unit of Kf (Chen et al. 1999). The Ku values (L/g) of Zn adsorption onto Fe-WTR and Al-WTR (from lowest to highest imposed concentrations) ranged 0.70–0.15 and 0.69–0.21, respectively, while Ku values for Pb adsorption onto Fe-WTR ranged 1.65–1.06 L/g (Table 4).
From the Temkin model, the equilibrium constant AT values of Zn adsorption onto Fe-WTR and Al-WTR were 3.50 l/g and 3.32 l/g, respectively, and the R2(adj) value was 0.97 for both, while AT values of Pb adsorption onto Fe-WTR and Al-WTR were 6.50 l/g and 2.99 l/g, respectively, and the R2(adj) values were 0.77 and 0.74, respectively (Table 3). The standard Gibbs free energy of the adsorption can be calculated using Eq. 5. Based on the Temkin constant AT, the standard Gibbs free energy of Zn adsorption onto Fe-WTR and Al-WTR was − 13.24 and − 13.11 kJ/mol, respectively, and of Pb adsorption onto Fe-WTR and Al-WTR was − 17.56 and − 15.67 kJ/mol, respectively. These results indicate that Pb adsorption has more negative values and is thus more easily adsorbed on WTRs than Zn, which supports the results described above in that the more H-shaped isotherms for Pb indicated that saturation was not approached.
Competitive adsorption/desorption of Zn-Pb ions
Removal efficiency from solution was 100% for Pb and almost 100% (> 98%) for Zn (Table 5), revealing that WTRs had high capacity for sorbing both metals when present together but also demonstrating a marginally higher affinity for Pb sorption by Al-WTR and Fe-WTR compared with Zn ions that was statistically significant. A similar pattern was evident in the desorption findings in which no Pb was detectably released from either WTR type, while only a very small percentage of adsorbed Zn (0.03–3.92%) was released, thus also indicating a marginally higher affinity and/or binding retention of Pb of the cationic adsorption sites of the WTRs than that for Zn ions (Table 5).
Desorption of Pb and Zn (single element)
The desorption percentages (i.e. the percentage of adsorbed metal that was extracted by 0.001 M CaCl2) of both Pb and Zn were very low for all samples for both types of WTRs (Table 6). For example, desorption percentages from WTRs originally exposed to 400 mg/l metal solutions were 0.23 ± 0.06% and 1.26 ± 0.03% for Pb and Zn, respectively, for the Al-WTRs. Samples of Al-WTRs subjected to the lowest initial concentration of Pb and Zn, i.e. 10 mg/l, had desorption percentage values of 6.24 ± 1.23% and 7.10 ± 2.77% for Pb and Zn, respectively (Table 6). For the Fe-WTRs, desorption percentages for samples subjected to initial concentrations of Pb and Zn of 400 mg/l were 0.00 ± 0.00% and 0.36 ± 0.06%, respectively. For Fe-WTR samples subjected to the lowest initial concentration of Pb and Zn, i.e. 10 mg/l, the desorption percentages were 0.02 ± 0.00% and 2.24 ± 1.56% for Pb and Zn, respectively (Table 6). This again shows that both types of WTRs have high capacity for binding and retaining metals. Extraction of WTR constituent metals by the sorption and desorption solutions was also negligible, in keeping with previous experiments in our laboratory on these WTRs that showed Al, Fe and other metals were not appreciatively mobile unless the pH was forced below 5 (Howells et al. 2018).
Discussion
Adsorption and desorption
The levels of sorption demonstrated here (~ 4000 mg/kg, without reaching saturation) provide definitive values that add to the growing literature on WTR sorption capacity and strength (e.g. Chiang et al. 2012; Zhou and Haynes 2011; Silvetti et al. 2015). From the Langmuir isotherm, which fit the data well for Zn sorption (but not for Pb), the maximum monolayer coverage capacity (Qm) of the Zn adsorption onto Fe-WTR and Al-WTR could be calculated as 4.38 mg/g and 4.68 mg/g, respectively, which is greater than that reported for comparable recovered materials such as activated carbon (1.23 mg/g; Üҫer et al. 2006), hazelnut shells (1.78 mg/g; Cimino et al. 2000) and treated pine bark (1.18 mg/g; Vazquez et al. 1994) but lower than that reported for coal fly ash (11.11 mg/g; Pehlivan et al. 2006). One could speculate that sorption beyond this theoretical monolayer maximum may be achieved through continued coagulation and flocculation effects on the metal ions in solution by the oxide and hydroxide (OH−) surfaces of the WTRs through indirect hydrogen bonding forces, which have been shown to be important in comparable situations (Dash et al. 2011). Any hydroxide-bearing colloids released by the WTRs could also have a similar effect. It is interesting that the Al-WTRs and the Fe-WTRs both performed equally at sorbing Zn, whereas the study by Chiang et al. (2012) found that Fe-WTRs outperformed Al-WTRs in this regard. One could speculate that this inconsistency across studies might reflect wider differences between the plants from where Chiang and partners obtained their WTRs compared to those plants from where WTRs were obtained for the present study, i.e. wider differences in raw water characteristics and/or differences in supporting electrolytes or levels of lime added during water treatment, which could have made a greater difference between their WTRs beyond just that created from the use of different primary coagulants.
The Zn adsorption isotherms (Fig. 2) clearly indicated a high affinity of both WTR materials for this metal. However, although there was a very high adsorption efficiency across the entire range of concentrations imposed (always > 93%, Table 2), there was a relative decrease in the adsorption efficiency at higher concentrations which, based on the Temkin model, might be attributed to a process in which the heat of adsorption of all the molecules in the layer decreases linearly with an increase in the coverage of WTRs surface due to adsorbent–adsorbate interactions (Bonilla-Petriciolet et al. 2017). Or, as implied by the Type I curves for Zn (Fig. 2), the decreased adsorption efficiency observed could simply be due to a decrease in the remaining available adsorbing sites as the amount of surface-bound Zn increased. It is also possible that a combination of these factors was at work. The Type I isotherms also indicate a pattern of adsorption that is consistent with that of microporous materials with narrow pores that limit multilayer adsorption (Lowell et al. 2012), which can consequently limit the overall amount adsorbed. The nature of the pores within WTRs was examined in part by Makris et al. (2004) who noticed that the carbon dioxide gas adsorption (232 pm width) was greater than that of N2 (370 pm width), suggesting steric restriction of N2 diffusion by narrow micropore openings. If WTRs typically have micropores of this scale, steric restriction might inhibit multilayer adsorption to some degree because of the size of Zn-hydrated ionic radii.
Contrastingly, the more H-type adsorption isotherm curves for Pb, particularly on Al-WTRs, indicated low surface saturation coverages (e.g. Qe/Qm < 0.1). This suggests that for Pb the WTRs had a great reserve capacity for further adsorption. It is known that Pb has more affinity for organic matter than does Zn, based on a typical affinity sequence of organic matter for metals (McBride 1989); thus, it is plausible that the organic matter content of the WTRs (28% and 26% by mass, respectively) played an important role in the higher Pb sorption capacity observed here. Nevertheless, because the organic matter percentage of the respective WTRs was so similar it is unlikely that variation in the organic matter content can account for the different shaped isotherms with respect to Pb sorption (i.e. the Al-WTRs had a classic H shape, whereas the Fe-WTRs had a more intermediate shape). A chemical precipitation/fixation process might thus have been involved for Pb on Al-WTRs. However, as high adsorption was observed with both WTRs used in the present study it is possible that a chemical precipitation might contribute to the high removal of both Pb and Zn in the case of both WTR types in addition to the adsorption processes. In relation to that, it is worth noting that based on values of standard electrode potential E° for Pb and Al in an electrochemical series, reduction reactions of the Pb could possibly occur in the presence of Al. Such a process would result in tight binding of Pb to the surface that would not be easily dislodged (i.e. strong retention).
Using the Temkin isotherm model (which was the best fitting model for adsorption of Zn onto WTRs as well as for Pb onto Fe-WTRs), the free energy of the sorption process can be calculated. That is, applying the Temkin adsorption constant in the classical Van’t Hoff equation can calculate standard Gibbs free energy for the process. Standard Gibbs free energy (ΔG°) values for adsorption of Zn onto Fe-WTR and Al-WTR were − 13.24 and − 13.11, respectively, while for adsorption of Pb onto Fe-WTR and Al-WTR were − 17.56 and − 15.67, respectively. Being minus (−) in sign, this indicates that the sorption processes would occur spontaneously and further emphasises that the sorption processes were thermodynamically favourable.
In terms of the slight preference for Pb sorption over Zn observed, it may be that the ions with the greatest charge density (ratio of charge to ionic radius) or the most electronegative are first adsorbed, and if there are still available sites, then the lower charge density or lower electronegative ion is adsorbed in sequence. This would fit with the known electronegativities for the studied metal ions, i.e. Pb(2.33) > Zn(1.65). Also, when a hydrated ion is subjected to electrostatic interactions, the rate of the solvent exchange between the hydration shells of an ion and the bulk of the water is determined by the ligand field stabilisation and the electric field of the ion. The electric field determines the rate of the solvent exchange in a way that the larger the field strength, the slower the exchange (Marcus 1988; Burgess 1999). Knowing that the charge density around Zn2+ is greater than that around Pb2+, the exchange of solvent (H2O) around Pb will be faster than that for Zn and this is another potential explanatory factor for the adsorption of the Pb being slightly more than that for Zn.
Desorption of Pb and Zn
The very low desorption rates, and thus strong retention of Pb and Zn, observed in this study suggest that not only outer-sphere (electrostatic bonding) complexes form between solute metals and WTRs but also inner-sphere complexes (ionic or covalent bonding directly with surface functional groups), the assertion of which is certainly further supported by the H-shaped isotherms for Pb (Sparks 2003). Quantitatively important inner-sphere complexes have been noted for adsorption of Pb to Al, Fe and Mn (hydr)oxides as well as for Zn sorption to Al, Fe and Mn (hydr)oxides in the literature (e.g. Trainor et al. 2000; Matocha et al. 2001), further supporting this idea. It is also known that in the presence of a ligand such as SO42− or CO32− a ternary complex can occur in which the ligand is between the surface functional group and the metal, acting as a bridge. When this occurs, the solubilities of metals and ligand drop below those expected from either adsorption or precipitation alone (Roberts et al. 2005); thus, the ligands present in the WTRs could also play a role in enhancing the sorption and retention. Adsorption of Pb onto goethite and of Zn onto alumina powder was notably enhanced by the presence of ligand bridges through formation of inner-sphere bidentate binding via ternary complexes (Ostergren et al. 2000; Trainor et al. 2000); thus, it is also a real possibility within the suite of mechanisms by which WTRs sorb and retain metals. Application of scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) techniques to WTRs recovered from batch sorption experiments such as those conducted in the present study may be able to provide more definitive information on the bonding mechanisms involved, as such steps have been successfully implemented elsewhere in studies of other sorbing materials (Wang et al. 2021; Zhu et al. 2021); future research with WTRs should incorporate this.
Conclusion
This investigation has shown that the two WTRs tested, Al-based and Fe-based, had high adsorption capacity for Pb and Zn both separately and in combination, with > 93% of each of the metals in solution removed at every concentration tested. Through examination of fits to the data of multiple isotherms, it was demonstrated that multiple sorption mechanisms are likely involved in the sorption of metals by WTRs. Moreover, the WTRs retained the vast majority of the adsorbed metals even through a vigorous desorption process, confirming their potential for environmental applications in which immobilisation or removal of metals is desirable.
References
Babatunde AO, Zhao YQ (2007) Constructive approaches toward water treatment works sludge management: an international review of beneficial reuses. Crit Rev Environ Sci Technol 37:129–164
Basibuyuk M, Kalat D (2004) The use of waterworks sludge for the treatment of vegetable oil refinery industry wastewater. Environ Technol 25:373–380
Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Avila HE (2017) Adsorption processes for water treatment and purification. Springer, Berlin
Burgess J (1999) Ions in solution: basic principles of chemical interactions. Elsevier, Amsterdam
Chen Z, Xing B, Mcgill W (1999) A unified sorption variable for environmental applications of the Freundlich equation. J Environ Qual 28:1422–1428
Chiang YW, Ghyselbrecht K, Santos RM, Martens JA, Swennen R, Cappuyns V, Meesschaert B (2012) Adsorption of multi-heavy metals onto water treatment residuals: Sorption capacities and applications. Chem Eng J 200–202:405–415
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(Vi) from aqueous solution by hazelnut shell. Water Res 34:2955–2962
Coles CA, Yong RN (2006) Use of equilibrium and initial metal concentrations in determining Freundlich isotherms for soils and sediments. Eng Geol 85:19–25
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45
Dash M, Dwari RK, Biswal SK, Reddy PSR, Chattopadhyay P, Mishra BK (2011) Studies on the effect of flocculant adsorption on the dewatering of iron ore tailings. Chem Eng J 173:318–325
Howe KJ, Crittenden JC, Hand DW, Trussell RR, Tchobanoglous G (2012) Principles of water treatment. Wiley, Hoboken
Howells AP, Lewis SJ, Beard DB, Oliver IW (2018) Water treatment residuals as soil amendments: examining element extractability, soil porewater concentrations and effects on earthworm behaviour and survival. Ecotoxicol Environ Saf 162:334–340
Ippolito JA, Barbarick KA, Elliott HA (2011) Drinking water treatment residuals: a review of recent uses. J Environ Qual 40:1–12
Keeley J, Jarvis P, Judd SJ (2014) Coagulant recovery from water treatment residuals: a review of applicable technologies. Crit Rev Environ Sci Technol 44:2675–2719
Kim Y, Kim C, Choi I, Rengaraj S, Yi J (2004) Arsenic removal using mesoporous alumina prepared via a templating method. Environ Sci Technol 38:924–931
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. Innovation 2:100076
Lowell S, Shields JE, Thomas MA, Thommes M (2012) Characterization of porous solids and powders: surface area, pore size and density. Springer, Berlin
Makris KC, El-Shall H, Harris WG, O’connor GA, Obreza TA (2004) Intraparticle phosphorus diffusion in a drinking water treatment residual at room temperature. J Colloid Interface Sci 277:417–423
Marcus Y (1988) Ionic radii in aqueous solutions. Chem Rev 88:1475–1498
Matocha CJ, Elzinga EJ, Sparks DL (2001) Reactivity of Pb (II) at the Mn (III, IV)(oxyhydr) oxide—water interface. Environ Sci Technol 35:2967–2972
Mcbride MB (1989) Reactions controlling heavy metal solubility in soils. Adv Soil Sci 10:1–47
OECD (2000) Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method.
Ostergren JD, Brown GE Jr, Parks GA, Persson P (2000) Inorganic ligand effects on Pb (II) sorption to goethite (α-FeOOH): II. Sulfate. J Colloid Interface Sci 225:483–493
Pehlivan E, Cetin S, Yanik BH (2006) Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J Hazard Mater 135:193–199
Prakash P, Sengupta AK (2003) Selective coagulant recovery from water treatment plant residuals using donnan membrane process. Environ Sci Technol 37:4468–4474
Roberts D, Nachtegaal M, Sparks D L (2005) Speciation of metals in soils. In: Chemical Processes in Soils. Madison, WI. Soil Science Society of America
Silvetti M, Castaldi P, Garau G, Demurtas D, Deiana S (2015) Sorption of cadmium (II) and zinc (II) from aqueous solution by water treatment residuals at different pH values. Water Air Soil Pollut 226:313
Sparks DL (2003) Environmental Soil Chemistry, 2nd edn. Academic Press, Cambridge
Trainor TP, Brown GE Jr, Parks GA (2000) Adsorption and precipitation of aqueous Zn (II) on alumina powders. J Colloid Interface Sci 231:359–372
Turner T, Wheeler R, Stone A, Oliver I (2019) Potential alternative reuse pathways for water treatment residuals: remaining barriers and questions—a review. Water Air Soil Pollut 230:227
Üҫer A, Uyanik A, Aygun SF (2006) Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Sep Purif Technol 47:113–118
Vazquez G, Antorrena G, Gonzalez J, Doval MD (1994) Adsorption of heavy metal ions by chemically modified Pinus pinaster bark. Biores Technol 48:251–255
Wang Z, Jia Y, Song W, Li X, Xu K, Wang Z (2021) Optimization of boron adsorption from desalinated seawater onto UiO-66-NH2/GO composite adsorbent using response surface methodology. J Clean Prod 300:126974
Yildirim EH (2006) Surface chemistry of solid and liquid interfaces. J Adhes 83:507–508
Zhou Y-F, Haynes RJ (2011) Removal of Pb (II), Cr (III) and Cr (VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut 215:631–643
Zhu Y, He X, Xu J, Fu Z, Wu S, Ni J, Hu B (2021) Insight into efficient removal of Cr(VI) by magnetite immobilized with Lysinibacillus sp JLT12: mechanism and performance. Chemosphere 262:127901
Acknowledgement
The authors gratefully acknowledge funding from the Ministry of Higher Education and Scientific Research, Iraq (B859).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing interests or conflicts of interest. The authors have permission from Keele University to publish this work.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arab, K.A.H., Thompson, D.F. & Oliver, I.W. Evaluation and characterisation of metal sorption and retention by drinking water treatment residuals (WTRs) for environmental remediation. Int. J. Environ. Sci. Technol. 19, 7727–7736 (2022). https://doi.org/10.1007/s13762-021-03674-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03674-8