Abstract
Release and dispersion of toxic substances like heavy metals in environment have adverse effects on human health and other living organisms. Therefore, contaminant removal prior to discharging them into environmental is so crucial. This study aimed to investigate the efficiency of magnetic Fe3O4 nanoparticles coated with natural clinoptilolite zeolite (Fe3O4@Z) for cadmium removal from aqueous solution. In this study, the effect of operational parameters such as pH, contact time, nanocomposite dosages, initial cadmium concentration, and temperature on cadmium adsorption was investigated. In addition, adsorption equilibrium, kinetics, modeling, and thermodynamic parameters were analyzed. Maximum cadmium adsorption was observed at pH = 6 and the contact time of 60 min. By increasing the adsorbent dosage from 0.2 to 1 g/L, removal efficiency increased from 24.4.1 to 68.9% for 20 mg/L Cd concentration. Also, Cd removal followed Freundlich isotherm with R2 = 0.997 and pseudo-second-order kinetic model with R2 = 0.992. Modeling showed that reciprocal quadratic (R2 = 0.9594), rational function (R2 = 0.9996), and polynomial fit (R2 = 0.9990) models represented the best fitness for changes of cadmium removal efficiency with changes of pH, adsorbent dose, and concentration, respectively. Based on the obtained results, Fe3O4@Z nanocomposite could be applied as an efficient adsorbent for cadmium removal from aquatic environments.
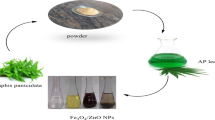
Graphical abstract







Similar content being viewed by others
References
Alqadami AA, Naushad M, Abdalla MA, Ahamad T, Alothman ZA, Alshehri SM, Ghfar AA (2017) Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J Clean Prod 156:426–436
Anwar J, Shafique U, Salman M, Dar A, Anwar S (2010) Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Biores Technol 101:1752–1755
Argun ME (2008) Use of clinoptilolite for the removal of nickel ions from water: kinetics and thermodynamics. J Hazard Mater 150:587–595
Baghapour MA, Mahvi AH, Pourfadakari S (2013) Thermodynamic analysis of reactive red 198 removal from synthetic wastewater by using multiwall carbon nanotubes. Health Scope 2:149–155
Bernhoft RA (2013) Cadmium toxicity and treatment. Sci World J 2013:1–7
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465
Deshmukh P, Khadse G, Shinde V, Labhasetwar P (2017) Cadmium removal from aqueous solutions using dried banana peels as an adsorbent: kinetics and equilibrium modeling. J Bioremediat Biodegrad 8:2
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Ghoneim MM, El-Desoky HS, El-Moselhy KM, Amer A, El-Naga EHA, Mohamedein LI, Al-Prol AE (2014) Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt J Aquat Res 40:235–242
Giwa A, Bello I, Oladipo M, Adeoye D (2013) Removal of cadmium from waste-water by adsorption using the husk of melon (Citrullus lanatus) Seed. Int J Basic Appl Sci 2:110–123
Gupta VK, Jain C, Ali I, Sharma M, Saini V (2003) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res 37:4038–4044
Inglezakis V, Loizidou M, Grigoropoulou H (2002) Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res 36:2784–2792
Isaac CPJ, Sivakumar A (2013) Removal of lead and cadmium ions from water using Annona squamosa shell: kinetic and equilibrium studies. Desalin Water Treat 51:7700–7709
Jahangirian H, Ismail MHS, Haron MJ, Rafiee-Moghaddam R, Shameli K, Hosseini S, Kalantari K, Khandanlou R, Gharibshahi E, Soltaninejad S (2013) Synthesis and characterization of Zeolite/Fe3O4 nanocomposite by green quick precipitation method. Dig J Nanomater Biostruct 4:4
Javadian H, Ghorbani F, Tayebi H-A, Asl SH (2015) Study of the adsorption of Cd(II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab J Chem 8:837–849
Jorfi S, Ahmadi MJ, Pourfadakari S, Jaafarzadeh N, Soltani RDC, Akbari H (2017) Adsorption of Cr (VI) by Natural clinoptilolite zeolite from aqueous solutions: isotherms and kinetics. Pol J Chem Technol 19:106–114
Kocasoy G, Şahin V (2007) Heavy metal removal from industrial wastewater by clinoptilolite. J Environ Sci Health Part A 42:2139–2146
Krika F, Azzouz N, Ncibi MC (2016) Adsorptive removal of cadmium from aqueous solution by cork biomass: equilibrium, dynamic and thermodynamic studies. Arab J Chem 9:S1077–S1083
Krishnan KA, Anirudhan T (2003) Removal of cadmium (II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: kinetics and equilibrium studies. Water SA 29:147–156
Mallakpour S, Behranvand V (2016) Polymeric nanoparticles: recent development in synthesis and application. Express Polym Lett 10:895
Murkani M, Nasrollahi M, Ravanbakhsh M, Bahrami P, Jaafarzadeh Haghighi Fard N (2015) Evaluation of natural zeolite clinoptilolite efficiency for the removal of ammonium and nitrate from aquatic solutions. Environ Health Eng Manage 2(1):17–22
Nalbandian MJ, Zhang M, Sanchez J, Choa Y-H, Nam J, Cwiertny DM, Myung NV (2016) Synthesis and optimization of Fe2O3 nanofibers for chromate adsorption from contaminated water sources. Chemosphere 144:975–981
Naushad M, Al-Othman Z, Islam M (2013) Adsorption of cadmium ion using a new composite cation-exchanger polyaniline Sn (IV) silicate: kinetics, thermodynamic and isotherm studies. Int J Environ Sci Technol 10:567–578
Naushad M, Ahamad T, Alothman ZA, Shar MA, Alhokbany NS, Alshehri SM (2015) Synthesis, characterization and application of curcumin formaldehyde resin for the removal of Cd2+ from wastewater: kinetics, isotherms and thermodynamic studies. J Ind Eng Chem 29:78–86
Olabemiwo FA, Tawabini BS, Patel F, Oyehan TA, Khaled M, Laoui T (2017) Cadmium removal from contaminated water using polyelectrolyte-coated industrial waste fly ash. Bioinorg Chem Appl 2017:1–13
Padilla-Ortega E, Leyva-Ramos R, Mendoza-Barron J (2014) Role of electrostatic interactions in the adsorption of cadmium (II) from aqueous solution onto vermiculite. Appl Clay Sci 88:10–17
Pasandideh EK, Kakavandi B, Nasseri S, Mahvi AH, Nabizadeh R, Esrafili A, Kalantary RR (2016) Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@ SiO2) for natural organic matter removal. J Environ Health Sci Eng 14:21
Pérez-Marín A, Zapata VM, Ortuno J, Aguilar M, Sáez J, Lloréns M (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater 139:122–131
Poinern GEJ, Brundavanam S, Tripathy SK, Suar M, Fawcett D (2016) Kinetic and adsorption behaviour of aqueous cadmium using a 30 nm hydroxyapatite based powder synthesized via a combined ultrasound and microwave based technique. Phys Chem 6:11–22
Pourfadakari S, Mahvi AH (2014) Kinetics and equilibrium studies for removal of Reactive Red 198 from aqueous solutions using zero valent iron powder. Health Scope 3:1–8
Pourfadakari S, Jorfi S, Ahmadi M, Takdastan A (2017) Experimental data on adsorption of Cr (VI) from aqueous solution using nanosized cellulose fibers obtained from rice husk. Data Brief 15:887–895
Raji C, Anirudha T (1997) Chromium (VI) adsorption by sawdust carbon: kinetics and equilibrium. India J Chem Technol 4:228–236
Rezaei Kalantry R, Jonidi Jafari A, Esrafili A, Kakavandi B, Gholizadeh A, Azari A (2016) Optimization and evaluation of reactive dye adsorption on magnetic composite of activated carbon and iron oxide. Desalin Water Treat 57:6411–6422
Ruyter-Hooley M, Johnson BB, Morton DW, Angove MJ (2017) The adsorption of myo-inositol hexaphosphate onto kaolinite and its effect on cadmium retention. Appl Clay Sci 135:405–413
Satarug S, Vesey DA, Gobe GC (2017) Kidney cadmium toxicity, diabetes and high blood pressure: the perfect storm. Tohoku J Exp Med 241:65–87
Shaheen SM, Derbalah AS, Moghanm FS (2012) Removal of heavy metals from aqueous solution by zeolite in competitive sorption system. Int J Environ Sci Dev 3:362
Takdastan A, Pourfadakari S, Jorfi S (2018) Ultrasonically induced adsorption of nitrate from aquoeos solution using Fe3O4@ activated carbon nanocomposite. Desalin Water Treat 123:230–239
Tang C, Shu Y, Zhang R, Li X, Song J, Li B, Zhang Y, Ou D (2017) Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv 7:16092–16103
Tesi GO (2014) Sorption and desorption studies on toxic metals from brewery effluent using eggshell as adsorbent. Adv Nat Sci 7:15–24
Wang Q, Kanel SR, Park H, Ryu A, Choi H (2009) Controllable synthesis, characterization, and magnetic properties of nanoscale zerovalent iron with specific high Brunauer–Emmett–Teller surface area. J Nanopart Res 11:749–755
Yanagisawa H, Matsumoto Y, Machida M (2010) Adsorption of Zn(II) and Cd(II) ions onto magnesium and activated carbon composite in aqueous solution. Appl Surf Sci 256:1619–1623
Zheng W, Li X-M, Wang F, Yang Q, Deng P, Zeng G-M (2008) Adsorption removal of cadmium and copper from aqueous solution by areca—a food waste. J Hazard Mater 157:490–495
Zhu C, Luan Z, Wang Y, Shan X (2007) Removal of cadmium from aqueous solutions by adsorption on granular red mud (GRM). Sep Purif Technol 57:161–169
Zoleikani L, Issazadeh H, Zarenezhad B (2015) Preparation of new conductive polymer nanocomposites for cadmium removal from industrial wastewaters. J Chem Technol Metall 50:71–80
Acknowledgments
This work was supported by Student Research Committee, Ahvaz Jundishapur University of Medical Sciences (Grant No. ETRC- 94S120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Gaurav Sharma.
Rights and permissions
About this article
Cite this article
Jorfi, S., Shooshtarian, M.R. & Pourfadakari, S. Decontamination of cadmium from aqueous solutions using zeolite decorated by Fe3O4 nanoparticles: adsorption modeling and thermodynamic studies. Int. J. Environ. Sci. Technol. 17, 273–286 (2020). https://doi.org/10.1007/s13762-019-02350-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02350-2