Abstract
Purpose
Mesial temporal lobe epilepsy (mTLE) is a chronic focal epileptic disorder characterized by recalcitrant seizures often necessitating surgical intervention. Identifying the laterality of seizure focus is crucial for pre-surgical planning. We implemented diffusion MRI (DMRI) connectometry to identify differences in white matter connectivity in patients with left and right mTLE relative to healthy control subjects.
Method
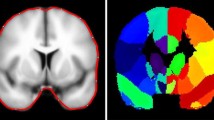
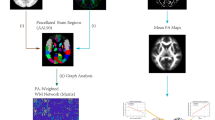
We enrolled 12 patients with right mTLE, 12 patients with left mTLE, and 12 age/sex matched healthy controls (HCs). We used DMRI connectometry to identify local connectivity patterns of white matter tracts, based on quantitative anisotropy (QA). We compared QA of white matter to reconstruct tracts with significant difference in connectivity between patients and HCs and then between patients with left and right mTLE.
Results
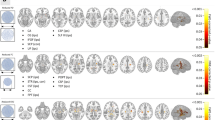
Right mTLE patients show higher anisotropy in left inferior longitudinal fasciculus (ILF) and forceps minor and lower QA in genu of corpus callosum (CC), bilateral corticospinal tracts (CSTs), and bilateral middle cerebellar peduncles (MCPs) compared to HCs. Left mTLE patients show higher anisotropy in genu of CC, bilateral CSTs, and right MCP and decreased anisotropy in forceps minor compared to HCs. Compared to patients with right mTLE, left mTLE patients showed increased and decreased connectivity in some major tracts.
Conclusions
Our study showed the pattern of microstructural disintegrity in mTLE patients relative to HCs. We demonstrated that left and right mTLE patients have discrepant alternations in their white matter microstructure. These results may indicate that left and right mTLE have different underlying pathologic mechanisms.


Similar content being viewed by others
References
Engel J Jr.(1996) Introduction to temporal lobe epilepsy. Epilepsy Res 26(1):141–150
Gross DW (2011) Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52(Suppl 4):32–34
Dumas de la Roque A, Oppenheim C, Chassoux F, Rodrigo S, Beuvon F, Daumas-Duport C et al (2005) Diffusion tensor imaging of partial intractable epilepsy. Eur Radiol 15(2):279–285
Bonilha L, Martz GU, Glazier SS, Edwards JC (2012) Subtypes of medial temporal lobe epilepsy: influence on temporal lobectomy outcomes? Epilepsia 53(1):1–6
Labate A, Cherubini A, Tripepi G, Mumoli L, Ferlazzo E, Aguglia U et al (2015) White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia 56(7):1109–1116
Widjaja E, Geibprasert S, Otsubo H, Snead OC, Mahmoodabadi SZ (2011) Diffusion tensor imaging assessment of the epileptogenic zone in children with localization-related epilepsy. AJNR Am J Neuroradiol 32(10):1789–1794
Zhao F, Kang H, You L, Rastogi P, Venkatesh D, Chandra M (2014) Neuropsychological deficits in temporal lobe epilepsy: a comprehensive review. Ann Indian Acad Neurol 17(4):374–382
Salehi F, Sharma M, Peters TM, Khan AR (2017) White matter tracts in patients with temporal lobe epilepsy: pre- and postoperative assessment. Cureus 9(10):e1735
Yeh F-C, Badre D, Verstynen T. Connectometry (2016) A statistical approach harnessing the analytical potential of the local connectome. NeuroImage 125:162–171. https://doi.org/10.1101/136473
Yeh F-C, Tang P-F, Tseng W-YI (2013) Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage Clin 2:912–921
Sobhani S, Rahmani F, Aarabi MH, Sadr AV (2017) Exploring white matter microstructure and olfaction dysfunction in early parkinson disease: diffusion MRI reveals new insight. Brain Imaging Behav. https://doi.org/10.1007/s11682-017-9781-0
Leemans A, Jeurissen B, Sijbers J, Jones DK (2009) ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: Proceedings of the 17th scientific meeting, International Society for Magnetic Resonance in Medicine, Honolulu, p 3537
Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran J-P et al (2014) Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. NeuroImage Clin 5:349–358
Ahmadi ME, Hagler DJ Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM et al (2009) Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol 30(9):1740–1747
Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M et al (2014) Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage 100:135–144
Nazem-Zadeh M-R, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M et al (2016) DTI-based response-driven modeling of mTLE laterality. NeuroImage Clin 11:694–706
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29(1):196–205
Yeh FC, Badre D, Verstynen T. Connectometry (2016) A statistical approach harnessing the analytical potential of the local connectome. NeuroImage 125:162–171
Yeh FC, Panesar S, Fernandes D, Meola A, Yoshino M, Fernandez-Miranda JC (2018) Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 178:57–68
Rahmani F, Sobhani S, Aarabi MH (2017) Sequential language learning and language immersion in bilingualism: diffusion MRI connectometry reveals microstructural evidence. Exp Brain Res 235(10):2935–2945
Correa DG, Pereira M, Zimmermann N, Doring T, Ventura N, Rego C et al. (2018) Widespread white matter DTI alterations in mesial temporal sclerosis independent of disease side. Epilepsy Behav 87:7–13
Zhang T, Kong J, Jing K, Chen H, Jiang X, Li L, Guo L, Lu J, Hu X, Liu T (2015) Multi-scale and multimodal fusion of tract-tracing, myelin stain and DTI-derived fibers in macaque brains. In: Navab N, Hornegger J, Wells W, Frangi A (eds) Medical image computing and computer-assisted intervention: MICCAI international conference on medical image computing and computer-assisted intervention. Springer, Cham, pp 246–254
Nazem-Zadeh MR, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M et al (2016) DTI-based response-driven modeling of mTLE laterality. NeuroImage Clin 11:694–706
Coan AC, Campos BM, Yasuda CL, Kubota BY, Bergo FP, Guerreiro CA et al (2014) Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One 9(1):e85843
Helmstaedter C, May TW, von Lehe M, Pfaefflin M, Ebner A, Pannek HW et al (2014) Temporal lobe surgery in Germany from 1988 to 2008: diverse trends in etiological subgroups. Eur J Neurol 21(6):827–834
Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu YC (2016) Age effects and sex differences in human brain white matter of young to middle-aged adults: a DTI, NODDI, and q-space study. NeuroImage 128:180–192
Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X et al (2011) Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum Brain Mapp 32(6):883–895
Govindan RM, Makki MI, Sundaram SK, Juhász C, Chugani HT (2008) Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res 80(1):30–41
Lin JJ, Riley JD, Juranek J, Cramer SC (2008) Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res 82(2):162–170
Chaudhary UJ, Duncan JS (2014) Applications of blood-oxygen-level-dependent functional magnetic resonance imaging and diffusion tensor imaging in epilepsy. Neuroimaging Clin N Am 24(4):671–694
Sone D, Sato N, Kimura Y, Watanabe Y, Okazaki M, Matsuda H (2018) Brain morphological and microstructural features in cryptogenic late-onset temporal lobe epilepsy: a structural and diffusion MRI study. Neuroradiology 60(6):635–641
Doucet GE, Pustina D, Skidmore C, Sharan A, Sperling MR, Tracy JI (2015) Resting-state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Hum Brain Mapp 36(1):288–303
Su L, An J, Ma Q, Qiu S, Hu D (2015) Influence of resting-state network on lateralization of functional connectivity in mesial temporal lobe epilepsy. AJNR Am J Neuroradiol 36(8):1479–1487
Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J Jr, Stern JM (2014) Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55(1):137–145
Ashtari M (2011) Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol 54(1):6–7
Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain A J Neurol 136(6):1692–1707
Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NCR et al (2018) Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci 38(8):2106–2117
Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP (2012) A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 53(4):659–667
Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N (2002) Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: Effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 73(6):648–656
Bernhardt BC, Worsley KJ, Besson P, Concha L, Lerch JP, Evans AC et al (2008) Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: Insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage 42(2):515–524
Zeng H, Pizarro R, Nair VA, La C, Prabhakaran V (2013) Altered regional homogeneity in mesial temporal lobe epilepsy patients with hippocampal sclerosis. Epilepsia 54(4):658–666
Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O et al (2011) Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res 1373:221–229
Govindan RM, Makki MI, Sundaram SK, Juhasz C, Chugani HT (2008) Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res 80(1):30–41
Whelan CD, Alhusaini S, O’Hanlon E, Cheung M, Iyer PM, Meaney JF et al (2015) White matter alterations in patients with MRI-negative temporal lobe epilepsy and their asymptomatic siblings. Epilepsia 56(10):1551–1561
Wieshmann UC, Milinis K, Paniker J, Das K, Jenkinson MD, Brodbelt A et al (2015) The role of the corpus callosum in seizure spread: MRI lesion mapping in oligodendrogliomas. Epilepsy Res 109:126–133
Caligiuri ME, Labate A, Cherubini A, Mumoli L, Ferlazzo E, Aguglia U et al (2016) Integrity of the corpus callosum in patients with benign temporal lobe epilepsy. Epilepsia 57(4):590–596
Keller SS, Ahrens T, Mohammadi S, Gerdes JS, Moddel G, Kellinghaus C et al (2013) Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. J Neuroimaging 23(3):352–359
McDonald CR, Hagler DJ Jr, Ahmadi ME, Tecoma E, Iragui V, Dale AM et al (2008) Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy Res 79(2–3):130–138
Szabo CA, Lancaster JL, Lee S, Xiong JH, Cook C, Mayes BN et al (2006) MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol 27(10):2155–2160
Kreilkamp BAK, Weber B, Richardson MP, Keller SS (2017) Automated tractography in patients with temporal lobe epilepsy using TRActs Constrained by UnderLying Anatomy (TRACULA). NeuroImage Clin 14:67–76
Barrick TR, Lawes IN, Mackay CE, Clark CA (2007) White matter pathway asymmetry underlies functional lateralization. Cereb Cortex 17(3):591-598
Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S et al (2005) Asymmetry analysis of cingulum based on scale-invariant parameterization by diffusion tensor imaging. Hum Brain Mapp 24(2):92–98
Shon YM, Kim YI, Koo BB, Lee JM, Kim HJ, Kim WJ et al (2010) Group-specific regional white matter abnormality revealed in diffusion tensor imaging of medial temporal lobe epilepsy without hippocampal sclerosis. Epilepsia 51(4):529–535
Acknowledgement
This study was supported in part by NIH grant R01EB013227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval of the study was granted by the Institutional Review Board at Henry Ford Health System, Detroit, MI, USA.
Informed consent
No informed consent was needed because anonymized data were used in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hossein Sanjari Moghaddam, Farzaneh Rahmani and Mohammad Hadi Aarabi have equal contributions to this work.
Rights and permissions
About this article
Cite this article
Sanjari Moghaddam, H., Rahmani, F., Aarabi, M. et al. White matter microstructural differences between right and left mesial temporal lobe epilepsy. Acta Neurol Belg 120, 1323–1331 (2020). https://doi.org/10.1007/s13760-019-01074-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-019-01074-x