Abstract
Key message
N addition (56, 156, and 206 kg N ha−1 yr−1as dissolved NH4NO3) method (canopy vs soil) did not affect the biomass of N2-fixers (Acacia mangium Willd. and Ormosia pinnata Lour.), but significantly affected the biomass of non-N2-fixers (Schima superba Gardner & Champ., Pinus massoniana Lamb.). Coniferous species exposed to N addition on the canopy rather than the soil had higher N accumulation.
Context
Previous experiments simulating nitrogen (N) addition in forests were conducted by adding N fertilizer directly to soils, which neglects the fact that N uptake can be done by canopy leaves.
Aims
The objective of this study is to examine how different N addition methods (canopy vs soil) affect growth and N accumulation of four subtropical tree seedlings.
Methods
An open-air greenhouse experiment was conducted to expose four tree species (Schima superba Gardner & Champ., Pinus massoniana Lamb., Acacia mangium Willd. and Ormosia pinnata Lour.) to different N addition methods (canopy or soil) and N levels (ambient, medium, or high).
Results
N addition method affected the biomass of non-N2-fixers (Schima superba Gardner & Champ. and Pinus massoniana Lamb.), while N2-fixers (Acacia mangium Willd. and Ormosia pinnata Lour.) were unaffected. N concentrations in the soils and leaves of all trees were significantly increased by the medium and high N additions, and soil N concentrations resulted from N addition via soil rather than the canopy. Although leaf N concentration was significantly affected by N addition method in all trees except for Ormosia pinnata, only N accumulation in Pinus massoniana was significantly affected by N addition method.
Conclusion
N addition method affected the biomass of non-N2-fixers and N accumulation in coniferous species, while it did not affect the biomass of N2-fixers and N accumulation in broadleaf species.
Similar content being viewed by others
1 Introduction
An excess of 10 kg nitrogen (N) ha−1 yr.−1 deposition has been detected in 11% of the world’s natural vegetation (Pardo et al. 2011). China is a rapidly developing country in Asia that is also experiencing intensifying N deposition in its central and southeastern areas (Xu et al. 2015; Zhu et al. 2015). High N deposition within the range 30–73 kg N ha−1 yr.−1 was observed in forests in Southern China (Mo et al. 2006; Zhao et al. 2017). N deposition below a critical threshold can increase the foliar N and photosynthetic capacity of tree species (Bauer et al. 2004; Fleischer et al. 2013); however, N deposition above the threshold directly damages leaf tissues (Liu et al. 2007) and inhibits tree growth (Lu et al. 2010, 2014). Excess N deposition can often increase soil acidity, which causes nutrient loss from the soil (Bowman et al. 2008; Lu et al. 2014, 2015) and a decrease in plant diversity (Hogberg et al. 2006; Bobbink et al. 2010).
Numerous studies have reported how N deposition affects plant growth (Lu et al. 2010; Liu et al. 2017). However, almost all the experiments simulating N deposition have been conducted by adding N solution and/or fertilizer directly onto the soil (forest floor). Conventional experiments with addition of N to the understory largely neglect the fact that nutrient uptake can be done directly by canopy leaves and/or twigs (Adriaenssens et al. 2012; Fernández and Brown 2013; Guerrieri et al. 2015; Chater and Garner 2018), and therefore may not realistically simulate atmospheric N deposition or generate reliable impacts on forest ecosystems (Zhang et al. 2015). It is well known that natural N deposition passes through the canopy layer before it reaches the forest floor. When N is deposited directly on the canopy, it becomes immediately available to plant leaves and subsequently stimulates photosynthesis and increases production (Wortman et al. 2012). However, when N solution and/or fertilizer is added directly onto the soil, it affects plant growth indirectly via soil acidification and nutrient loss (Bowman et al. 2008; Lu et al. 2014). Hence, the effects of N addition via plant leaves and soils on plant growth should be different. Therefore, it is necessary to understand the effects of different N addition methods on plant growth.
Responses of tree growth to N addition are often species-specific (Elvir et al. 2006; Liu et al. 2017; Mao et al. 2017). Elvir et al. (2006) found that Acer saccharum Marshall, but not Fagus grandifolia Ehrh. and Pinus resinosa Sol. ex Aiton, showed significantly higher photosynthetic rates after a decade of N inputs. Mao et al. (2017) found that the highly light-dependent species responded significantly to N addition, but medium-light and shade-tolerant species did not in a tropical forest. Mo et al. (2008) reported that net photosynthetic rate of two tree seedlings (Schima superba Gardner & Champ. and Cryptocarya concinna Hance) varied in response to simulated N deposition depending on the rate of N addition and species-specific N requirements. Recently, Liu et al. (2017) demonstrated that two N2 fixers grew significantly faster than two non-N2 fixers in response to N addition in subtropical China.
Here, we examined the N accumulation and plant growth of four tree species, including two N2-fixers and two non-N2-fixers, in a greenhouse in response to three N addition levels (ambient, medium, and high) and two addition methods (canopy and soil) in subtropical China. We hypothesized that: (1) the responses of tree growth in N-rich tropical areas to N addition are species-specific; (2) different N addition methods have different effects on plant growth and N accumulation in the four tree species, and (3) the stimulation of N accumulation is greater in canopy N addition than soil N addition as plant leaves can directly take-up the added N from the canopy.
2 Materials and methods
2.1 Experimental set-up
The study was conducted in an open-air greenhouse at the South China Botanical Garden in Guangzhou city, Guangdong Province, China (23°20′ N, 113°30′ E). The mean annual temperature, precipitation, and relative humidity were 21.5 °C, 1750 mm, and 77%, respectively. In March 2012, 144 plastic pots (40-cm diameter, 30-cm height) were prepared. Each pot was filled with Ultisol soil. The soil was collected from a nearby evergreen broad-leaved forest. The pH for the selected soil was 4.00 ± 0.10. Soil organic matter was 22.72 ± 1.20 g kg−1. In the soil, the NO3−N and NH4+-N were 17.39 ± 1.34 mg kg−1 and 6.01 ± 0.69 mg kg−1, respectively. One-year-old seedlings of four species were collected from a nursery and transplanted into the pots (one seedling per pot) avoiding root damage. Among the four species, Ormosia pinnata Lour. and Acacia mangium Willd. are N2-fixers, while Schima superba and Pinus massoniana Lamb. are non-N2-fixers. In the two non-N2-fixers, S. superba is a broad-leaf species and P. massoniana is a needle species. These species are common tree species in southern China.
On 10 April 2012, the 144 pots were arranged in six randomized complete blocks. Each block was made of six different N treatments, with four plant species per treatment, for a total of 24 pots per block. The six N treatments were: S-CK (ambient N addition to the soil), S-MN (medium N addition to the soil), S-HN (high N addition to the soil), C-CK (ambient N addition to the canopy), C-MN (medium N addition to the canopy), and C-HN (high N addition to the canopy). As the ambient wet N deposition was about 56 kg ha−1 yr.−1 in the South China Botanical Garden in 2006 (Liu et al. 2008), we set the three N levels at 56, 156, and 206 kg ha−1 yr.−1, for the ambient, medium, and high levels, respectively. NH4NO3-N was dissolved in tap water and then slowly added to the seedling canopies by spraying or directly onto pot soil once a week. For the canopy addition, we first covered the soil in each pot with clingfilm, and then sprayed the N solution slowly from the top of the seedling using a mist sprinkler. We aimed to cover all branches. No other fertilizer was used. All pots received the same amount of water.
2.2 Sample collection and measurement
To measure initial soil parameters, soil samples were collected from the 0 to 20 cm layer in each pot before the seedlings were transplanted. In June and December 2012 and April, July, and November 2013, soil samples were collected from the 0 to 20 cm layer in each pot to measure the concentrations of NO3−-N and NH4+-N in the soil. Each soil sample was a pool sampled from three soil cores (inner diameter: 2.5 cm), and was stored in a hard, plastic container for transport to the laboratory. Soil samples were air-dried and sieved using a 2-mm mesh. Dead roots and plant residues were removed. Soil pH was determined using a glass electrode in the supernatants by shaking for 2 h and sedimentation in a beaker for 24 h in deionized CO2-free water. The soil to H2O ratio was 1:2.5. Soil organic C was determined following the Walkley-Black wet digestion method (Nelson and Sommers 1982). The soil NO3−N was determined using a modified ultraviolet-visible (UV) spectrophotometry method from Norman et al. (1985). The soil NO3−-N was extracted using a 1 M KCl solution with a 1:5 soil to solution ratio. Then, the mixture was shaken at 20 ± 2 °C for 1 h in an oscillator. After filtration through Whatman no. 1 filter paper, the leachate was then determined at 220 nm and 275 nm, respectively. The measurement principle of the method was based on the difference in the spectral adsorption properties between dissolved organic carbon (DOC) and nitrate. NO3−-N has the strongest absorption of UV light at 220 nm wavelength and the weakest absorption at 275 nm. DOC absorbs light at all wavelengths between 200 and 300 nm. In this study, NO3-N concentration was obtained by measuring the absorbance of the KCl extract at 220 nm and 275 nm wavelengths. More detailed information is given in Song et al. (2007). To measure soil NH4+-N, the soil was also extracted using a 1 M KCl solution with a 1:5 soil to solution ratio. However, in this case, the KCl extract was determined at 625 nm using the indophenol blue spectrophotometric method (Horn and Squire 1966).
To measure soil phospholipid fatty acid (PLFA), soil samples were also collected from each pot in April and October 2013. Three soil cores (2.5 cm in diameter) were randomly chosen from the 0 to 20 cm layer and homogenized into one sample per pot. Soil samples were put into sealed plastic bags and transported to the laboratory immediately. Roots, stones, and other materials were removed; each soil sample was screened through a 2-mm sieve and stored at 4 °C until analysis. Detailed information about soil PLFA measurements are given in Liu et al. (2017).
In March, June, and December 2012 and April, July, and November 2013, about 6–10 new fully expanded leaves from each seedling were randomly sampled to analyze N concentration. All leaves were carefully rinsed and air dried, and then dried at 70 °C in an oven for 72 h, then finely ground (0.25 mm). Foliar N concentration was measured using the Kjeldahl method (Bremner and Mulvaney 1982). To measure tree biomass, all seedlings were harvested by carefully digging them out of the soil in November 2013. The biomass (B, g dry matter) of fine roots (diameter ≤ 2 mm), coarse roots (diameter > 2 mm), stems, and leaves in each harvested seedling were obtained after oven-drying at 60 °C for 1 day. The accumulation of N was calculated as follows:
2.3 Statistical analysis
Shapiro-Wilk’s test was used to test the normality and homogeneity of variance before statistical analysis. The effects of N addition level, N addition method, and their interaction on tree growth and N accumulation were analyzed using a linear mixed model. The effects of N addition, N addition method, sampling time, and their interactions on N concentrations in both soil and leaf samples were analyzed using repeated measures analysis of variance with a linear mixed model. Effects of N addition level and addition method on the microbial PLFAs were examined using a general linear model. When there was a significant effect of N addition level or method, data were further analyzed using Tukey’s test. Differences were considered as statistically significant at p < 0.05. SAS software (SAS Institute Inc., Cary, NC, USA) was used for the data analysis.
3 Results
3.1 Biomass
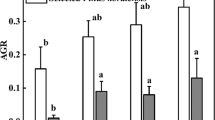
N addition levels, addition methods, and their interaction all significantly affected tree root biomass, but the effect was species-specific (Fig. 1). High N addition via the plant canopy significantly increased (p < 0.05) fine root biomass of A. mangium and P. massoniana. However, only medium N addition via soil significantly increased (p < 0.05) fine root biomass of A. mangium. High N addition via soil significantly decreased (p < 0.05) fine root biomass of O. pinnata and P. massoniana.
Effects of N addition levels and methods on total and fine root biomass in four tree species (mean ± SD). Different lowercase letters above the error bars indicate significant differences among N addition level in each species. S-CK: ambient N addition to the soil, S-MN: medium N addition to the soil, S-HN: high N addition to the soil, C-CK: ambient N addition to the canopy, C-MN: medium N addition to the canopy, and C-HN: high N addition to the canopy. Data of soil N addition are from Liu et al. (2017)
Both medium and high N addition significantly increased (p < 0.05) the biomass of A. mangium compared with the ambient N addition, irrespective of N addition method (Fig. 1). High N addition did not affect the biomass of O. pinnata. Only medium N addition via soil significantly increased (p < 0.05) the biomass of S. superba. High N addition via plant canopy significantly increased (p < 0.01) the biomass of P. massoniana, while both high and medium N addition via soil significantly decreased (p < 0.01) the biomass of P. massoniana (Fig. 1), which suggests that the N addition method significantly affected (p < 0.05) the biomass of this needle species. Overall, N addition method significantly affected (p < 0.01) the biomass of non-N2-fixers (S. superba and P. massoniana), while N2-fixers (A. mangium and O. pinnata) were unaffected.
3.2 Leaf stoichiometry
Leaf N concentrations in all species increased over time. Both high and medium N addition resulted in higher leaf N concentrations in all the species (p < 0.005) (Fig. 2). N addition method significantly affected (p ≤ 0.01) leaf N concentration in all species, except O. pinnata. Higher leaf N concentrations were found in A. mangium when they received N addition via the soil, rather than the canopy. However, the opposite was observed in P. massoniana, as higher leaf N concentrations were shown in the pots that received N addition via the canopy (p < 0.001).
Effects of N addition levels, methods, and time on leaf N concentrations in four tree species. S-CK: ambient N addition to the soil, S-MN: medium N addition to the soil, S-HN: high N addition to the soil, C-CK: ambient N addition to the canopy, C-MN: medium N addition to the canopy, and C-HN: high N addition to the canopy
3.3 N accumulation
High N addition via the plant canopy significantly increased (p < 0.01) N accumulation in A. mangium, S. superba, and P. massoniana (Fig. 3). Both high and medium N addition via soil significantly increased (p < 0.01) N accumulation in the N2-fixers (A. mangium and O. pinnata), while both high and medium N addition via soil significantly decreased (p < 0.01) N accumulation in P. massoniana (Fig. 3). Overall, N addition method did not affect N accumulation in any of the tree species, except for P. massoniana. Higher N accumulation was observed in P. massoniana exposed to canopy rather than soil N addition.
Effects of N addition levels and methods on N accumulation in four tree species (mean ± SD). Different lowercase letters above the error bars indicate significant differences among N addition level in each species (p < 0.05). S-CK: ambient N addition to the soil, S-MN: medium N addition to the soil, S-HN: high N addition to the soil, C-CK: ambient N addition to the canopy, C-MN: medium N addition to the canopy, and C-HN: high N addition to the canopy
3.4 Soil N concentrations
Soil NH4+-N concentrations were lower than NO3−-N in all species (Tables 1 and 2). High N addition significantly increased (p < 0.05) NH4+-N concentrations in all species, irrespective of N addition method. Higher NH4+-N concentrations were found in the pots that received N addition via soil. Sampling time significantly affected soil NH4+-N concentrations.
Compared with the ambient N addition, high N addition significantly increased (p < 0.05) NO3−-N concentration in the soil of pots of all species. N addition method significantly affected soil NO3−-N concentration, with higher values in the pots that received N addition via soil. Among the four species, the soil in P. massoniana pots showed the highest NO3−-N concentration, followed by the soil in the A. mangium and S. superba pots. The soil in O. pinnata pots had the lowest NO3−-N concentration (Table 2).
3.5 Phospholipid fatty acids (PLFAs)
Higher N addition significantly decreased microbial PLFAs in the soil of all the species pots, irrespective of the N addition method. In April 2013, N addition significantly decreased (p < 0.05) Arbuscular mycorrhizal fungi (AMF) in the soil in all species, irrespective of the N addition method. However, in October 2013, N addition significantly decreased (p < 0.05) the PLFAs in all pots, except for those in the soil around S. superba (Table 3). Meanwhile, N addition via soil significantly decreased PLFAs in the soil around O. pinnata and S. superba in October 2013. In both sampling periods, N addition method did not affect soil microbial PLFAs in the pots with A. mangium, O. pinnata, or P. massoniana. However, for S. superba, higher soil microbial PLFAs were found in the pots that received canopy rather than soil N addition (p < 0.05).
4 Discussion
N2-fixers show a significant increase in yield in response to N addition when N2 fixation is unable to meet plant N demand (Salvagiotti et al. 2008; Thies et al. 1995). The growth stimulation in N2-fixers in response to N addition was also observed in our study. Medium and high N addition significantly increased the biomass of A. mangium. However, high N addition did not affect the total biomass of O. pinnata. Some previous studies also showed that N addition had no effect on the biomass production of N2-fixers (Xia and Wan 2008; Zhang et al. 2011), as high N addition inhibited symbiotic nitrogenase activity in some legumes (Lee et al. 2003; Thomas et al. 2000; Zhang et al. 2011). Different responses of A. mangium and O. pinnata to N additions indicated that the effects of N addition on N2-fixers were species-specific. N addition method did not affect the total biomass of N2-fixers, but significantly affected the biomass of non-N2-fixers (S. superba and P. massoniana). This might have been in part because N addition method did not affect soil microbial PLFAs of N2-fixers in our study. N2-fixers are able to fix N2 to meet plant N demand, which probably explains the lack of growth response to the different N addition methods. Our results support our first hypothesis that the responses of tree growth in N-rich tropical areas to N addition method are species-specific.
In all species, high and medium additions of N improved the concentration of N in the leaves. This was a consequence of the increased input of N fertilizer with the treatments. N addition methods significantly affected leaf N concentrations in A. mangium and P. massoniana. Higher leaf N concentrations were found in A. mangium that received N addition via the soil rather than the canopy. However, the opposite effects were observed in P. massoniana. This indicated that responses of plant N accumulation to different N addition methods were species-specific. Fernández and Eichert (2009) also found that the response to foliar nutrient sprays was variable among species. Foliar spray uptake is determined by plant characteristics such as leaf shape and chemistry, and stomata and physical attributes including cuticle composition and surface wax architecture (Fernández et al. 2008; Fernández and Brown 2013). The structure and composition of the cuticle and cuticular waxes vary greatly between plant species (Heredia-Guerrero et al. 2008; Leide et al. 2007). The obvious difference in leaf shape and physical properties among the tree species in this study might explain the varied response to nutrient canopy sprays. We found that N addition method only affected the N accumulation ability of P. massoniana. Higher N accumulation was observed in P. massoniana exposed to canopy rather than soil N addition. Coniferous needles are covered by long-chain, aliphatic epicuticular waxes (Wytteenbach et al. 1987; Cape and Percy 1998), which might induce high needle N uptake. The occurrence of biological nitrification in coniferous needle and the high N retention by twigs of coniferous needle when the needle was exposed to high N deposition might also lead to high needle N uptake (Adriaenssens et al. 2012; Guerrieri et al. 2015). Compared to the other species with broad-leaves, the needles of P. massoniana can directly take-up more nutrients, which led to higher leaf N concentrations and greater biomass of this species after canopy N addition. Dail et al. (2009) also found that coniferous species showed high canopy retention of N rather than soils when they added 18 kg N ha−1 yr.−1 as dissolved NH4NO3 directly to the canopy of spruce-hemlock forest. We note that higher N accumulation was not shown in P. massoniana exposed to soil N addition in our study. Our results partly support our second hypothesis that different N addition methods have different effects on plant growth and N accumulation in the four tree species. N addition method did not affect N accumulation in A. mangium, O. pinnata, or S. superba, which was not consistent with our hypothesis that the stimulation of nutrient accumulation was greater after canopy N addition than soil N addition.
Soil microorganisms are essential for nutrient cycling in terrestrial ecosystems. Previous studies have shown that high N deposition has a negative influence on soil microbial communities (LeBauer and Treseder 2008; Treseder 2008; Wallenstein et al. 2006). Treseder (2008) conducted a meta-analysis and found that microbial biomass declined 15% on average due to N addition, and that the declines in abundances of microbes were more obvious with longer durations and higher total amounts of N addition. Our results are consistent with these findings. Compared to the control, high and medium N additions significantly reduced microbial PLFAs in the soil of all pots in our experiment, irrespective of N addition method. High N addition decreased microbial PLFAs more than medium N addition. During the experimental period, N addition method did not affect soil microbial PLFAs in A. mangium, O. pinnata, or P. massoniana pots. However, higher soil microbial PLFAs were found in the pots of S. superba that received N addition via the canopy rather than the soil. When the soil was treated with a high N level, soil microorganisms were directly affected, which resulted in lower soil microbial PLFAs in the pots planted with S. superb. As our experiment lasted only 1.5 years, we did not detect the effects of soil N addition on the soil microbial PLFAs of the other three species. We suspect that N addition via soil would have had stronger effects on microorganisms than N addition via the canopy in a long-term experiment of the other three species. N addition via soil did not affect soil microbial PLFAs for either of the N2-fixers (A. mangium and O. pinnata) over the study period. This indicated that N2-fixers have a higher ability to resist the negative influences of high N addition than non-N2-fixers in tropical areas. Among the four species, the soil in P. massoniana pots showed the highest NO3−-N concentration. A higher N accumulation was shown in P. massoniana exposed to canopy N addition than soil N addition, which suggested that needle species accumulate nutrients largely via the leaves.
Our knowledge of N deposition impacts on forest plants mainly derives from numerous field manipulation experiments. Almost all the previous experiments simulating N deposition have been conducted by adding N fertilizer directly onto the understory plants or forest soils. However, natural atmospheric N deposition often arrives on the canopy before the soil. Thus, conventional experiments neglect many components and ecological processes in the canopy that are critical for forest plants. Hence, further experiments simulating N deposition by adding N fertilizer to the canopy are necessary. In our study, we added N fertilizer via both canopy and soil and showed that the effects of different N addition methods on plant growth and N accumulation were species-specific. Therefore, N addition method should be accounted for in future N deposition experiments. As the pots used in our experiment were limited in volume and all seedlings grew very fast, our experiment only lasted about 1.5 years. N deposition over the short term often stimulates plant growth and nutrient uptake in tropical tree species (Liu et al. 2017; Mao et al. 2017); however, some studies also showed that plant growth does not generally respond to N fertilization as soils in many humid tropical forests are already N-rich (Cusack et al. 2011, 2016; Kaspari et al. 2008). As the effect of N addition on soil microorganisms over the short term is also different to that over the long term, long-term and continuous field experiments are necessary to further improve our understanding on the effects of different N addition methods on plant growth in tropical areas.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adriaenssens S, Staelens J, Wuyts K, Samson R, Verheyen K, Boeckx P (2012) Retention of dissolved inorganic nitrogen by foliage and twigs of four temperate tree species. Ecosystems 15:1093–1107
Bauer GA, Bazzaz FA, Minocha R, Long S, Magill A, Aber J, Berntson GM (2004) Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in the NE United States. For Ecol Manag 196:173–186
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59
Bowman AW, Peters RC, Roelofs JG (2008) Long term changes in atmospheric N and S throughfall deposition and effects on soil solution chemistry in a Scots Pine Forest in the Netherlands. Environ Pollut 156:1252–1259
Bremner J, Mulvaney C (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2: chemical and microbial properties. Agronomy monograph, vol 9. Agronomy Society of America, Madison, pp 595–624
Cape JN, Percy KE (1998) Use of needle epicuticular wax chemical composition in the early diagnosis of Norway spruce (Picea abies (L.) Karst.) decline in Europe. Chemosphere 36:895–900
Chater JM, Garner LC (2018) Foliar nutrient applications to ‘wonderful’ pomegranate (Punica granatum L.). II. Effects on leaf nutrient status and fruit split, yield and size. Sci Hortic 242:207–213
Cusack DF, Silver WL, Torn MS, McDowell WH (2011) Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry 104:203–225
Cusack DF, Macy J, McDowell WH (2016) Nitrogen additions mobilize soil base cations in two tropical forests. Biogeochemistry 128:67–88
Dail DB, Hollinger DY, Davidson EA, Fernandez I, Sievering HC, Scott NA, Gaige E (2009) Distribution of nitrogen-15 tracers applied to the canopy of a mature spruce-hemlock stand, Howland, Maine, USA. Oecologia 160:589–599
Elvir J, Wiersma G, Day M, Greenwood MS, Fernandez IJ (2006) Effects of enhanced nitrogen deposition on foliar chemistry and physiological processes of forest trees at the Bear Brook Watershed in Maine. For Ecol Manag 221:207–214
Fernández V, Brown PH (2013) From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front Plant Sci 4:289
Fernández V, Eichert T (2009) Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit Rev Plant Sci 28:36–68
Fernández V, Eichert T, Del Rio V, Lopez-Casado G, Heredia-Guerrero JA, Abadia A, Heredia A, Abadia J (2008) Leaf structural changes associated with iron deficiency chlorosis in field-grown pear and peach: physiological implications. Plant Soil 311:161–172
Fleischer K, Rebel KT, van der Molen MK, Erisman JW, Wassen MJ, van Loon EE, Mon tagnani L, Gough CM, Herbst M, Janssens IA (2013) The contribution of nitrogen deposition to the photosynthetic capacity of forests. Glob Biogeochem Cycles 27:187–199
Guerrieri R, Vanguelova EI, Michalski G, Heaton THE, Mencuccini M (2015) Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies. Glob Chang Biol 21:4613–4626
Heredia-Guerrero JA, Benítez JJ, Heredia A (2008) Self-assembled polyhydroxy fatty acids vesicles: a mechanism for plant cutin synthesis. BioEssays 30:273–277
Hogberg P, Fan HB, Quist M, Binkley D, Tamm CO (2006) Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob Chang Biol 12:489–499
Horn DB, Squire CR (1966) The estimation of ammonia using the indophenol blue reaction. Clin Chim Acta 14:185–194
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Lee TD, Tjoelker MG, Reich PB, Russelle MP (2003) Contrasting growth response of an N2-fixing and non-fixing forb to elevated CO2: dependence on soil N supply. Plant Soil 255:475–486
Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G (2007) The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol 144:1667–1679
Liu JX, Zhou GY, Yang CW, Qu ZY, Peng CL (2007) Responses of chlorophyll fluorescence and xanthophyll cycle in leaves of Schima superba Gardn. & Champ and Pinus massoniana Lamb. to simulated acid rain at Dinghushan Biosphere Reserve, China. Acta Physiol Plant 29:33–38
Liu JX, Zhang DQ, Zhou GY, Faivre-Vuillin B, Deng Q, Wang CL (2008) CO2 enrichment increases nutrient leaching from model forest ecosystems in subtropical China. Biogeosciences 5:1783–1795
Liu JX, Li YY, Xu Y, Liu SE, Huang WJ, Fang X, Yin GC (2017) Phosphorus uptake in four tree species under nitrogen addition in subtropical China. Environ Sci Pollut Res 24:20005–20014
Lu XK, Mo JM, Gilliam FS, Zhou GY, Fang YT (2010) Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob Chang Biol 16:2688–2700
Lu XK, Mao QG, Gilliam FS, Luo YQ, Mo JM (2014) Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob Chang Biol 20:3790–3801
Lu XK, Mao QG, Mo JM, Gilliam FS, Zhou GY, Luo YQ, Zhang W, Huang J (2015) Divergent responses of soil buffering capacity to long-term N deposition in three tropical forests with different land-use history. Environ Sci Technol 49:4072–4080
Mao QG, Lu XK, Wang C, Zhou KJ, Mo JM (2017) Responses of understory plant physiological traits to a decade of nitrogen addition in a tropical reforested ecosystem. For Ecol Manag 401:65–74
Mo JM, Brown S, Xue JH, Fang Y, Li Z (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests of subtropical China. Plant Soil 282:135–151
Mo JM, Li DJ, Gundersen P (2008) Seedling growth response of two tropical tree species to nitrogen deposition in southern China. Eur J For Res 127:275–283
Nelson D, Sommers L (1982) Carbon and organic matter. In: Page AL, Mille RH, Keeney DR (eds) Methods of soil analysis – part 2: chemical and microbiological properties. American Society of Agronomy, Madison, pp 561–579
Norman RJ, Edberg JC, Stucki JW (1985) Determination of nitrate in soil extract by dual-wavelength ultraviolet spectrophotometry. Soil Sci Soc Am J 49:1182–1185
Pardo LH, Fenn ME, Goodale CL, Geiser LH, Driscoll CT, Allen EB, Baron JS, Bobbink R, Bowman WD, Clark CM (2011) Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol Appl 21:3049–3082
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A (2008) Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crop Res 108:1–13
Song G, Sun B, Jiao JY (2007) Comparison between ultraviolet spectrophotometry and other methods in determination of soil nitrate-N. Acta Pedol Sin 44:288–293 (In Chinese with English Abstract)
Thies JE, Singleton PW, Bohlool BB (1995) Phenology, growth, and yield of field-grown soybean and bush bean as a function of varying modes of N nutrition. Soil Biol Biochem 27:575–583
Thomas RB, Bashkin MA, Richter DD (2000) Nitrogen inhibition of nodulation and N2 fixation of a tropical N2 fixing tree (Gliricidia sepium) grown in elevated atmospheric CO2. New Phytol 145:233–243
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manag 222:459–468
Wortman E, Tomaszewski T, Waldner P, Schleppi P, Thimonier A, Eugster W, Buchmann N, Sievering H (2012) Atmospheric nitrogen deposition and canopy retention influences on photosynthetic performance at two high nitrogen deposition Swiss forests. Tellus Ser B Chem Phys Meteorol 64:1
Wytteenbach A, Tobler L, Bajo S (1987) Deposition of aerosol on spruce needles and its chemical composition. J Aerosol Sci 18:609–612
Xia JY, Wan SQ (2008) Global response patterns of terrestrial plant species to nitrogen addition. New Phytol 179:428–439
Xu W, Luo XS, Pan YP, Zhang L, Tang AH, Shen JL, Zhang Y, Li KH, Wu QH, Yang DW, Zhang YY, Xue J, Li WQ, Li QQ, Tang L, Lu SH, Liang T, Tong YA, Liu P, Zhang Q, Xiong ZQ, Shi XJ, Wu LH, Shi WQ, Tian K, Zhong XH, Shi K, Tang QY, Zhang LJ, Huang JL, He CE, Kuang FH, Zhu B, Liu H, Jin X, Xin YJ, Shi XK, Du EZ, Dore AJ, Tang S, Collett JL Jr, Goulding K, Sun YX, Ren J, Zhang FS, Liu XJ (2015) Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China. Atmos Chem Phys 15:12345–12360
Zhang L, Wu DX, Shi HQ, Zhang CJ, Zhan XY, Zhou SX (2011) Effects of elevated CO2 and N addition on growth and N2 fixation of a legume subshrub (Caragana microphylla Lam.) in temperate grassland in China. PLoS One 6:e26842
Zhang W, Shen WJ, Zhu SD, Wan SQ, Luo YQ, Yan JH, Wang KY, Liu L, Dai HT, Li PX (2015) Can canopy addition of nitrogen better illustrate the effect of atmospheric nitrogen deposition on forest ecosystem? Sci Rep 5:11245
Zhao YH, Zhang L, Chen YF, Liu XJ, Xu W, Pan YP, Duan L (2017) Atmospheric nitrogen deposition to China: a model analysis on nitrogen budget and critical load exceedance. Atmos Environ 153:32–40
Zhu JX, He NP, Wang QF, Yuan GF, Wen D, Yu GR, Jia YL (2015) The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci Total Environ 511:777–785
Funding
This study was jointly funded by the National Natural Science Foundation of China (Grant Nos. 31570482, 31670487 and 41430529), Key Research Program of Frontier Sciences, CAS (Y621231001), and the Guangdong Hundred, Thousand, and Ten Thousand Talents Program.
Author information
Authors and Affiliations
Contributions
Project preparation: Liu JX. Experiment design: Huang WJ. Experiment work: Li YY nad Lie ZY. Data analysis: Lin W and Wu T. Manuscript draft: Liu JX and Wu T. Manuscript editing: All authors have studied and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Marco Ferretti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, T., Lin, W., Li, Y. et al. Nitrogen addition method affects growth and nitrogen accumulation in seedlings of four subtropical tree species: Schima superba Gardner & Champ., Pinus massoniana Lamb., Acacia mangium Willd., and Ormosia pinnata Lour. Annals of Forest Science 76, 23 (2019). https://doi.org/10.1007/s13595-019-0806-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-019-0806-2