Abstract
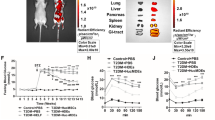
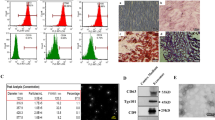
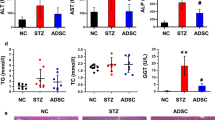
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs)-based therapy is currently considered to be an effective treatment for NAFLD. The present study aimed to determine whether hUC-MSCs-exosomes have a hepatoprotective effect on NAFLD. We constructed NAFLD rat model by high-fat high-fructose feeding. Liver cells (L-O2) were treated with palmitic acid (PA) to mimic NAFLD model. NAFLD rats and PA-treated L-O2 cells were treated with hUC-MSCs-exosomes, and then we determined the influence of exosomes on liver damage and glucose and lipid metabolism in vivo and in vitro. We found that hUC-MSCs-exosomes exhibited an up-regulation of miR-627-5p. Exosomal miR-627-5p promoted cell viability and repressed apoptosis of PA-treated L-O2 cells. Exosomal miR-627-5p also enhanced the expression of G6Pc, PEPCK, FAS and SREBP-1c and suppressed PPARα expression in PA-treated L-O2 cells. Moreover, miR-627-5p interacted with fat mass and obesity-associated gene (FTO) and inhibited FTO expression in L-O2 cells. MiR-627-5p-enriched exosomes improved glucose and lipid metabolism in L-O2 cells by targeting FTO. In vivo, exosomal miR-627-5p ameliorated insulin tolerance, liver damage, glucose and lipid metabolism and reduced lipid deposition in NAFLD rats. Exosomal miR-627-5p also reduced body weight, liver weight, and liver index (body weight/liver weight) in NAFLD rats. In conclusion, these data demonstrate that HUC-MSCs-derived exosomal miR-627-5p improves glucose and lipid metabolism and alleviate liver damage by repressing FTO expression, thereby ameliorating NAFLD progression. Thus, hUC-MSCs-exosomes may be a potential treatment for NAFLD.






Similar content being viewed by others
References
Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28(1):1–11. https://doi.org/10.1016/j.hepres.2003.08.001.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23. https://doi.org/10.1053/jhep.2003.50161.
de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48:S104–12. https://doi.org/10.1016/j.jhep.2008.01.009.
Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019;40(5):1367–93. https://doi.org/10.1210/er.2019-00034.
Chen L, Chen XW, Huang X, Song BL, Wang Y, Wang Y. Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci. 2019;62(11):1420–58. https://doi.org/10.1007/s11427-019-1563-3.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411(1):21–35. https://doi.org/10.1111/nyas.13435.
Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28(7):497–505. https://doi.org/10.1016/j.tem.2017.03.003.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–72. https://doi.org/10.1038/s41580-018-0044-8.
Li B, Cheng Y, Yu S, Zang L, Yin Y, Liu J, et al. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates nonalcoholic fatty liver disease in obese type 2 diabetic mice. Stem cells international. 2019;2019:8628027. https://doi.org/10.1155/2019/8628027.
Phinney DG, Pittenger MF. Concise Review: MSC-derived exosomes for cell-free therapy. Stem cells. 2017;35(4):851–8. https://doi.org/10.1002/stem.2575.
He Q, Wang L, Zhao R, Yan F, Sha S, Cui C, et al. Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res Ther. 2020;11(1):223. https://doi.org/10.1186/s13287-020-01731-6.
Zou XY, Yu Y, Lin S, Zhong L, Sun J, Zhang G, et al. Comprehensive miRNA analysis of human umbilical cord-derived mesenchymal stromal cells and extracellular vesicles. Kidney Blood Press Res. 2018;43(1):152–61. https://doi.org/10.1159/000487369.
Mizuno TM. Fat mass and obesity associated (FTO) gene and hepatic glucose and lipid metabolism. Nutrients. 2018;10(11):1600. https://doi.org/10.3390/nu10111600.
Hu Y, Feng Y, Zhang L, Jia Y, Cai D, Qian SB, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of mA on lipogenic mRNAs. RNA Biol. 2020;17(7):930–42. https://doi.org/10.1080/15476286.2020.1736868.
Hu J, Chen Y, Huang Y, Su Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Exp Mol Pathol. 2020;115: 104468. https://doi.org/10.1016/j.yexmp.2020.104468.
Yin Y, Hao H, Cheng Y, Zang L, Liu J, Gao J, et al. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018;9(7):760. https://doi.org/10.1038/s41419-018-0801-9.
Mizuno TM. FTOFat mass and obesity associated () gene and hepatic glucose and lipid metabolism. Nutrients. 2018;10(11):1600. https://doi.org/10.3390/nu10111600.
Shen M, Shen Y, Fan X, Men R, Ye T, Yang L. Roles of macrophages and exosomes in liver diseases. Front Med. 2020;7: 583691. https://doi.org/10.3389/fmed.2020.583691.
Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, et al. Lipotoxic hepatocyte-derived exosomal microRNA 192–5p activates macrophages through Rictor/Akt/Forkhead box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;72(2):454–69. https://doi.org/10.1002/hep.31050.
Li Y, Luan Y, Li J, Song H, Li Y, Qi H, et al. Exosomal miR-199a-5p promotes hepatic lipid accumulation by modulating MST1 expression and fatty acid metabolism. Hepatol Int. 2020;14:1–18. https://doi.org/10.1007/s12072-020-10096-0.
Sun F, Wu K, Yao Z, Mu X, Zheng Z, Sun M, et al. Long Noncoding RNA PVT1 promotes prostate cancer metastasis by increasing NOP2 expression via targeting tumor suppressor microRNAs. Onco Targets Ther. 2020;13:6755–65. https://doi.org/10.2147/ott.s242441.
Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W, et al. LINC00958 regulated miR-627-5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;20(9):1270–80. https://doi.org/10.1080/15384047.2019.1617571.
Wang J, Chen T, Wang L, Yao B, Sun L, Chen S, et al. MicroRNA-627-5p inhibits the proliferation of hepatocellular carcinoma cells by targeting BCL3 transcription coactivator. Clin Exp Pharmacol Physiol. 2020;47(3):485–94. https://doi.org/10.1111/1440-1681.13218.
Chen X, Gao Y, Yang X, Zhang H, Mo Z, Tan A. FTORelationship of gene variations with NAFLD risk in Chinese men. Open Life Sci. 2020;15(1):860–7. https://doi.org/10.1515/biol-2020-0081.
Zhang J, Li S, Li J, Han C, Wang Z, Li C, et al. Expression and significance of fat mass and obesity associated gene and forkhead transcription factor O1 in non-alcoholic fatty liver disease. Chin Med J. 2014;127(21):3771–6.
Chen A, Chen X, Cheng S, Shu L, Yan M, Yao L, et al. FTO promotes SREBP1c maturation and enhances CIDEC transcription during lipid accumulation in HepG2 cells. Biochim Biophys Acta. 2018;1863(5):538–48. https://doi.org/10.1016/j.bbalip.2018.02.003.
Chen J, Zhou X, Wu W, Wang X, Wang Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J Physiol Biochem. 2015;71(3):405–13. https://doi.org/10.1007/s13105-015-0420-1.
Stachowska E, Portincasa P, Jamioł-Milc D, Maciejewska-Markiewicz D, Skonieczna-Żydecka K. The Relationship between prebiotic supplementation and anthropometric and biochemical parameters in patients with NAFLD—a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(11):3460. https://doi.org/10.3390/nu12113460.
Acknowledgements
This study was supported by the National Key R&D Program of China, Synthetic Biology Research (No. 2019YFA0904500); the National Natural Science Foundation of China (No. 81860151); the Key R&D Program of Jiangxi Province (No. 20192BBG70027).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All protocols were authorized by the Ethics Committee of Second Affiliated Hospital of Nanchang University (No. NCDXSYDWFL-201067).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2021_593_MOESM1_ESM.tif
Supplementary Fig. 1 The body weight, liver weight, liver index and lipid deposition of NAFLD rats. Exosomes were isolated form hUC-MSCs following transfection of miR-627-5p inhibitor or inhibitor NC (miR-627-5pI-Exo, NCI-Exo). NAFLD rat model was constructed by HFHF feeding, followed by the exosome treatment. The body weight (A), liver weight (B) and liver index (C) of rats were measured. (D) The quantitative data of Oil Red O staining was presented. ***P < 0.001 vs. Normal; ##P < 0.01, ###P < 0.001 vs. Model; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. NCI-Exo (TIF 204 KB)
13577_2021_593_MOESM2_ESM.tif
Supplementary Fig. 2 The insulin levels of NAFLD rats. Exosomes were isolated form hUC-MSCs following transfection of miR-627-5p inhibitor or inhibitor NC (miR-627-5pI-Exo, NCI-Exo). NAFLD rat model was constructed by HFHF feeding, followed by the exosome treatment. The insulin levels of rats were detected by ELISA. ***P < 0.001 vs. Normal; ##P < 0.01 vs. Model; $P < 0.05 vs. NCI-Exo (TIF 118 KB)
Rights and permissions
About this article
Cite this article
Cheng, L., Yu, P., Li, F. et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Human Cell 34, 1697–1708 (2021). https://doi.org/10.1007/s13577-021-00593-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00593-1