Abstract
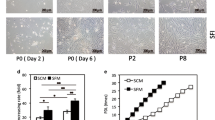
Mesenchymal stem cells (MSCs) have recently made significant progression in multiple clinical trials targeting several clinical disorders and in the modulation of immune responses. In the present study, we isolated human adipose tissue-derived stem cells (ADSCs) by direct membrane migration method without using enzymatic digestion via collagenase, and tried to extract adequate number of cells for clinical application. Hydroxyapatite-treated nonwoven fabric membrane made up of synthetic macromolecular fiber materials, polyethylene and polyester terephthalene was used. Expansion culture of ADSCs having plastic flask adherent characteristic in serum-free condition was successfully established, and adequate number of cells were obtained for clinical application. They were found to be positive for CD44, CD73, CD90 and CD105 and negative for CD11b, CD34, CD45, CD80 and HLA-DR. The resulting immunological marker profile satisfied the immunophenotype of previously reported MSCs. Also, microscopic findings demonstrated trilineage differentiation into adipogenic, osteogenic and chondrogenic cells as the characteristics of MSCs. The isolation by nonwoven fabric membrane and expanded cells under serum-free condition satisfied the criteria of MSCs, as proposed by the International Society for Cellular Therapy. Our direct membrane migration method without enzyme digestion is useful as ADSCs can be obtained from small pieces of adipose tissue and expanded under serum-free culture condition. This method was considered to be feasible for clinical application.






Similar content being viewed by others
References
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Raposio E, Caruana G, Petrella M, et al. A standardized method of isolating adipose-derived stem cells for clinical applications. Ann Plast Surg. 2016;76:124–6.
da Silva ML, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci Landmark. 2009;14:4281–98.
Wolf D, Wolf AM. Mesenchymal stem cells as cellular immunosuppressants. The Lancet. 2008;371:1553–4.
Doeppner TR, Hermann DK. Mesenchymal stem cells in the treatment of ischemic stroke: progress and possibilities. Stem Cells Cloning. 2010;3:157–63.
Liu Y, Lin L, Zou R, et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–22.
Tozawa K, Ono-Uruga Y, Yazawa M, et al. Megakaryocytes and platelets from a novel human adiposetissue–derived mesenchymal stem cell line. Blood. 2019;133:633–43.
Higuchi A, Wang CT, Ling QD, et al. A hybrid-membrane migration method to isolate high-purity adipose-derived stem cells from fat tissues. Sci Rep. 2015;5:10217.
Lin HR, Heish CW, Liu CH, et al. Purification and differentiation of human adipose-derived stem cells by membrane filtration and membrane migration methods. Sci Rep. 2017;7:40069.
https://www.bioind.com/israel/media/wysiwyg/documents/stem-cells/stem-cells-and-cell-therapy.pdf.
Yang Y, Lin H, Shen H, et al. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018;69:71–82.
Ougushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48(6):913–27.
Brown DV, Filiz G, Daniel PM, et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intratumor heterogeneity. PLoS ONE. 2017;12:e0172791.
Lee-Sayer SSM, Dougan MN, Cooper J, et al. CD44-mediated hyaluronan binding marks proliferating hematopoietic progenitor cells and promotes bone marrow engraftment. PLoS ONE. 2018;13:e0196011.
Khurana SS, Rieh TE, Moore BD, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–97.
Raposio E, Simonacci F, Perrotta RE. Adipose-derived stem cells: comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond). 2017;20:87–91.
Al-Saqi SH, Saliem M, Asikainen S, et al. Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy. 2014;16:915–26.
Tsuji K, Ojima M, Otabe K, Horie M, Koga H, Sekiya I, Muneta T. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 2017;26:1089–102.
Hendijani F. Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50:e12334.
Mitchell JB, Mcintosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell–associated markers. Stem Cells. 2006;24:376–85.
Vyas B, Shah A, Marathe A, et al. Adipose tissue: a natural resource for multipotent mesenchymal stem cells with potential translation to trigerminal layers. Indian J Plast Surg. 2018;51:177–81.
Dominici M, Blanc KL, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2016;8:315–7.
Acknowledgements
We thank Mr. Tatsuo Tanaka, Kyoto WakoPure Chemical Co., Ltd. Kyoto, Japan for his kind procession of hydroxyapatite treatment of non-woven fabric membrane.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. There is no grant of funding support for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuo, Y., Morita, H., Yamagishi, H. et al. Isolation of adipose tissue-derived stem cells by direct membrane migration and expansion for clinical application. Human Cell 34, 819–824 (2021). https://doi.org/10.1007/s13577-021-00505-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00505-3