Abstract
Introduction
The long-term benefit–risk profiles of licensed and investigational treatments for moderate-to-severe plaque psoriasis have not been fully characterized.
Methods
Randomized controlled trials (RCTs) of licensed and investigational treatments for moderate-to-severe plaque psoriasis were identified through a systematic literature review through 2 May 2021. Bayesian network meta-analyses (NMAs) were conducted to compare the efficacy (Psoriasis Area and Severity Index [PASI] 75/90/100 [at least a 75/90/100% reduction in PASI score from baseline] response) and safety outcomes (any adverse event [AE], any serious AE [SAE], and AEs leading to treatment discontinuation) of each treatment evaluated between weeks 48 and 56 after baseline. Surfaces under the cumulative ranking curves (SUCRAs) were calculated to evaluate the relative ranking of treatments. The benefit–risk profiles of treatments were assessed by bidimensional plots of the NMA-estimated efficacy and safety outcomes.
Results
In the efficacy NMA (N = 14 RCTs), the relative rankings for PASI 75/90/100 responses by weeks 48–56 were the highest for risankizumab (SUCRA: 98.5%) and bimekizumab (83.8% for dosing every 4 weeks [Q4W], 72.7% for dosing Q4W then every 8 weeks). The PASI response rates did not differ significantly between risankizumab and the two bimekizumab regimens. Additionally, risankizumab was associated with significantly higher PASI response rates than brodalumab, guselkumab, ixekizumab, secukinumab, ustekinumab, adalimumab, and etanercept. In the safety NMAs (N = 8 RCTs), risankizumab had the highest relative rankings for all three outcomes (SUCRA: 92.1%, 82.0%, and 91.0% for any AE, any SAE, and AEs leading to treatment discontinuation, respectively). Risankizumab had a significantly lower rate of any AE than bimekizumab, ustekinumab, and secukinumab.
Conclusions
Risankizumab was associated with the most favorable long-term benefit–risk profile for the treatment of moderate-to-severe plaque psoriasis. Although ixekizumab and bimekizumab had favorable efficacy profiles, both treatments had lower rankings for safety outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Prior research has established that the long-term benefits and risks associated with psoriasis treatments are important considerations during treatment decision-making. |
The long-term benefit–risk profiles of licensed and investigational treatments for moderate-to-severe plaque psoriasis have not been fully characterized. |
This study indirectly compared the long-term efficacy and safety outcomes of licensed and investigational treatments for moderate-to-severe plaque psoriasis based on network meta-analyses of clinical trial data and characterized their long-term benefit–risk profiles. |
What was learned from this study? |
Risankizumab and bimekizumab had the highest long-term Psoriasis Area and Severity Index response rates, and risankizumab had the lowest long-term rates of safety events. |
Although ixekizumab and bimekizumab had favorable efficacy profiles, both treatments had lower rankings for safety outcomes; thus, risankizumab was associated with the most favorable long-term benefit–risk profile for the treatment of moderate-to-severe plaque psoriasis. |
Introduction
Psoriasis, an inflammatory skin disease, is estimated to affect approximately 0.84% of the global population [1], with higher prevalence in Australasia, Europe, and North America [2, 3]. Symptoms of plaque psoriasis are multifactorial and vary in severity [4], necessitating a tailored approach to therapy. Mild-to-moderate plaque psoriasis can often be managed with topical therapies and phototherapy, while biologic agents have high efficacy for moderate-to-severe disease [5, 6]. Several novel biologic agents targeting the interleukin (IL)-17 pathway (ixekizumab, brodalumab, secukinumab) and the IL-23 pathway (risankizumab, guselkumab, tildrakizumab) have been recently approved by the United States (US) Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of moderate-to-severe plaque psoriasis. Furthermore, an investigational anti-IL-17 agent, bimekizumab, has shown promising data in several phase 3 clinical trials (BE VIVID, BE READY, BE SURE, and BE RADIANT) [7,8,9,10] and was recently licensed by the EMA [11].
As there is no cure for psoriasis, long-term treatments and ongoing disease management are critical to control psoriasis symptoms and related comorbidities [12,13,14,15]. Prior research has established that the long-term benefits and risks associated with psoriasis treatments are important considerations during treatment decision-making from both patients’ and physicians’ perspectives. For example, a systematic review by Sain et al. [16] reported that maintenance of treatment response was the most important attribute in several studies of the preferences of patients with psoriasis, and that the long-term risks of severe adverse events (AEs) such as the 10-year risks of tuberculosis, lymphoma, and serious infections were of primary concerns to both patients and physicians.
Data from both clinical trials and the real world have been routinely used to characterize and compare the long-term benefit–risk profiles of treatments for moderate-to-severe psoriasis. For example, Shear et al. [17] characterized the benefit–risk profiles of novel treatments by weeks 48–56 using a bidimensional plot of meta-analyzed long-term Psoriasis Area and Severity Index (PASI) response rates and long-term safety event rates based on network meta-analyses (NMAs). The study found that risankizumab was associated with the most favorable long-term benefit–risk profile compared with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab [17]. Additionally, a real-world retrospective multi-country, multicohort study by Torres et al. [18] compared drug survival, a composite metric capturing both efficacy and safety, among biologic agents for moderate-to-severe psoriasis. The authors found that the 18-month drug survival of the anti-IL-23 agents risankizumab and guselkumab was higher than that of ustekinumab, while that of secukinumab was lower versus ustekinumab [18]. In a systematic review and comparative meta-analysis of drug survival among biologics for psoriasis, Mourad and Gniadecki [19] found that ustekinumab was associated with longer drug survival than anti-tumor necrosis factor (TNF) and anti-IL-17 agents. However, there were insufficient data for guselkumab, the only anti-IL-23 agent considered in the study, to facilitate further comparisons.
The clinical decision-making process in the real world requires clinicians to evaluate multiple therapeutic options simultaneously. To inform this decision-making process, clinical trials provide a rich set of data on both licensed and investigational treatments, which can be synthesized using established indirect comparison methods to ensure a comprehensive comparison of the outcomes across treatments. In the present study, we indirectly compared the long-term efficacy and safety outcomes of licensed and investigational treatments for moderate-to-severe plaque psoriasis based on NMAs of clinical trial data and characterized their long-term benefit–risk profiles.
Methods
Data Source
Phase 2, 3, or 4 randomized controlled trials (RCTs) of licensed (by the US FDA or the EMA) and investigational treatments for moderate-to-severe plaque psoriasis were identified through a systematic literature review (SLR) through 2 May 2021. This SLR was originally conducted on 4 December 2017 and updated on 17 September 2018 (reported by Armstrong et al. [20]), 19 February 2021, and 2 May 2021. The searched databases include Embase, MEDLINE, and the Cochrane library. Additional searches were conducted for the reference lists of included studies, conference proceedings, previous health technology assessment submissions, and clinical trial registries.
The trial inclusion and exclusion criteria reported by Armstrong et al. [20], Armstrong et al. [21], and Shear et al. [17] were modified to accommodate both licensed and investigational treatments for moderate-to-severe plaque psoriasis. The trials were required to: (1) be a phase 2, 3, or 4 RCT for moderate-to-severe plaque psoriasis among adults who were eligible for systemic therapies or phototherapy; (2) include licensed treatments and dosages by the US FDA or the EMA or investigational treatments and dosages assessed in phase 3 clinical trials; and (3) report at least one of the efficacy outcomes (PASI 75, 90, and 100; indicating the proportions of patients who achieved at least a 75, 90, or 100% reduction in PASI score from baseline) or safety outcomes (any AEs, any serious AEs [SAEs], and AEs leading to treatment discontinuation) of interest by the end of the maintenance period (48–56 weeks from baseline). RCTs in which patients were crossed over from one treatment to another before the end of weeks 48–56, or were re-randomized based on certain efficacy criteria during the post-induction period, were excluded (for example, the VOYAGE-2 study was excluded because it rerandomized PASI 90 responders at week 28 to receive guselkumab or placebo [22]).
As this is a post-hoc NMA of previously published results of clinical trial data, no institutional board review was required. The study was performed in accordance with the Helsinki Declaration 1964 and its later amendments. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Comparators
The study included regimens for anti-IL-23 agents, anti-IL-17 agents, anti-TNF agents, and an anti-IL-12/23 agent through 48–56 weeks after randomization. The anti-IL-23 agents included guselkumab (100 mg at weeks 0 and 4, then every 8 weeks [Q8W]) and risankizumab (150 mg at weeks 0 and 4, then every 12 weeks [Q12W]). The anti-IL-17 agents included bimekizumab (320 mg every 4 weeks [Q4W] or 320 mg at weeks 0, 4, 8, 12, and 16, then Q8W); brodalumab (210 mg at weeks 0, 1, and 2, then every 2 weeks [Q2W]); ixekizumab (160 mg at week 0, 80 mg Q2W until week 12, then 80 mg Q4W); and secukinumab (300 mg at weeks 0, 1, 2, 3, and 4, then Q4W). The anti-TNF agents included adalimumab (80 mg at week 0, then 40 mg Q2W starting week 1) and etanercept (50 mg twice weekly [BIW] until week 12, then weekly [QW]). Finally, the anti-IL-12/23 agent was ustekinumab with a weight-based dosage (45 mg ≤ 100 kg, 90 mg > 100 kg at weeks 0 and 4, then Q12W).
Outcomes
The study examined both efficacy and safety outcomes by the end of the pre-specified maintenance period (weeks 48–56 after baseline). The efficacy outcomes were the proportions of patients with PASI 75, 90, and 100 responses; the safety outcomes were the proportions of patients who experienced any AE, any SAE, or AEs leading to treatment discontinuation.
Statistical Analyses
NMA Models
Separate NMA models were fitted for the efficacy and safety outcomes. Specifically, a fixed-effects Bayesian probit NMA was implemented to jointly model the PASI 75, 90, and 100 response rates, and fixed-effects Bayesian logistic NMAs were applied to individually model the rates of any AE, any SAE, and AEs leading to treatment discontinuation [23]. The fixed-effects models were selected because of the relative lack of long-term randomized controlled data that could be included into the network.
Based on the efficacy and safety NMA models, the proportions of patients achieving each of the efficacy and safety outcomes were described using posterior medians and 95% credible intervals (CrIs). Furthermore, the relative ranking of each treatment was assessed using the surface under the cumulative ranking curve (SUCRA) [24], a summary statistic ranging from 0% to 100%. In the efficacy NMA, a higher SUCRA value indicated that a treatment had a higher likelihood to have the highest PASI response rate among all treatments, i.e., the most efficacious treatment. In the safety NMAs, a higher SUCRA value suggested that a treatment had a higher likelihood to have the lowest safety event rates, i.e., the safest treatment. While the efficacy and safety of each treatment can be compared numerically using the posterior median for the proportion and the SUCRA value, neither measure can provide a formal statistical comparison between each pair of treatments. Therefore, odds ratios were used to formally compare the proportions between each pair of treatments and were summarized using posterior medians and 95% CrIs. Statistical significance was determined based on whether the 95% CrIs of odds ratios excluded 1.
Computation
A Markov Chain Monte Carlo (MCMC) procedure was used to draw the posterior samples for the Bayesian NMA models. The MCMC procedure was implemented with 5000 adaptation iterations, 50,000 burn-in iterations, and 50,000 posterior sampling iterations. Three parallel chains were run with a thinning factor of 10.
Benefit–Risk Profiles
Based on the NMAs, the benefit–risk profiles of each treatment were assessed through bidimensional plots of the SUCRA values of the PASI response rates and each of the safety outcomes. Treatments located at the top right corner of the graph were associated with the most favorable benefit–risk profiles.
R statistical software (version 3.6.2, the R Foundation for Statistical Computing, Vienna, Austria) and JAGS (version 4.3.0, Martyn Plummer, University of Warwick, Coventry, UK) were used for all statistical analyses.
Results
SLR and Study Selection
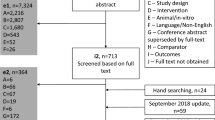
The SLR identified 689 publications for 215 RCTs through 2 May 2021 (Fig. 1). A total of 14 studies were included in the NMAs of long-term efficacy and safety outcomes, while 201 were excluded. The data extracted from the studies included in the long-term efficacy and safety NMAs are presented in Electronic Supplementary Material (ESM) Table S1.
Long-term Efficacy
A total of 14 RCTs connecting ten treatments were included in the efficacy NMA (Fig. 2a). The posterior median of the PASI response rates was the highest for risankizumab (84.9% for PASI 90 and 65.4% for PASI 100), bimekizumab 320 mg Q4W (81.3% and 59.9%, respectively), and bimekizumab Q4W then Q8W (79.4% and 57.3%, respectively), which were followed by brodalumab (78.6% and 56.1%, respectively), guselkumab (77.3% and 54.4%, respectively), ixekizumab (72.0% and 47.8%, respectively), secukinumab (66.2% and 41.3%, respectively), ustekinumab (55.1% and 30.5%, respectively), adalimumab (50.8% and 26.9%, respectively), and etanercept (37.4% and 16.9%, respectively).
Evidence network for the NMAs of PASI response (a) and safety events (b); any AE, any SAE, and AEs leading to treatment discontinuation) by the end of the maintenance period (weeks 48–56). a PASI response. The included trials were: AMAGINE-2 [39], AMAGINE-3 [39], ECLIPSE [40], VOYAGE-1 [41], CLEAR [42], FIXTURE [43], CLARITY [44], IXORA-S [45], UltIMMa1 [46], UltIMMa2 [46], IMMerge [37], BE VIVID [7], BE RADIANT [10], and BE SURE [9]. b Safety events (any AE, any SAE, and AEs leading to treatment discontinuation). The included trials were: ECLIPSE [40], VOYAGE-1 [41], CLEAR [47], CLARITY [44], IXORA-S [45], UltIMMa1 [46], UltIMMa2 [46], and BE RADIANT [10]. AE Adverse event, BIW twice weekly, PASI Psoriasis Area and Severity Index, QW once every week, Q4W once every 4 weeks, Q8W once every 8 weeks, SAE serious adverse event
Consistent with the PASI rankings, the SUCRA values were the highest for risankizumab (98.5%), bimekizumab Q4W (83.8%), and bimekizumab Q4W then Q8W (72.7%). The SUCRA values were the lowest for etanercept (0.1%), adalimumab (13.1%), and ustekinumab (20.1%) (Table 1).
There were no significant differences between the PASI response rates of risankizumab, bimekizumab Q4W, and bimekizumab Q4W then Q8W, with 95% probability (Table 2; ESM Table S2). However, risankizumab had significantly higher PASI response rates than brodalumab, guselkumab, ixekizumab, secukinumab, ustekinumab, adalimumab, and etanercept. Bimekizumab Q4W had significantly higher PASI response rates than ixekizumab, secukinumab, ustekinumab, adalimumab, and etanercept. Bimekizumab Q4W then Q8W, brodalumab, and guselkumab had significantly higher PASI response rates than secukinumab, ustekinumab, adalimumab, and etanercept (Table 2; ESM Table S2).
Long-term Safety
In the long-term safety NMAs, a total of eight trials for seven treatments were included (Fig. 2b).
Any AE
The posterior median for the rate of any AE was the lowest for risankizumab (67.5%), followed by guselkumab (72.2%), adalimumab (72.9%), secukinumab (76.6%), ustekinumab (76.9%), ixekizumab (80.9%), and bimekizumab (two dosages pooled) (82.3%). Consistent with these rankings, the SUCRA values were the highest for risankizumab (92.1%) and were the lowest for bimekizumab (two dosages pooled) (9.6%) and ixekizumab (22.0%) (Table 1).
Risankizumab had a significantly lower rate of any AE than secukinumab, ustekinumab, and bimekizumab (Table 3). Guselkumab had a significantly lower rate of any AE than bimekizumab. No other statistically significant differences were identified.
Any SAE
The posterior median for the rate of any SAE was the lowest for risankizumab (posterior median: 4.4%), followed by adalimumab (5.4%), ustekinumab (5.7%), guselkumab (5.9%), secukinumab (6.9%), bimekizumab (two dosages pooled) (7.2%), and ixekizumab (10.5%). Consistent with these rankings, the SUCRA values were the highest for risankizumab (82.0%) and the lowest for ixekizumab (16.5%) and bimekizumab (two dosages pooled) (36.2%) (Table 1). No statistically significant differences were identified (Table 3).
AEs Leading to Treatment Discontinuation
The posterior median for the rate of AEs leading to treatment discontinuation was the lowest for risankizumab (0.9%), followed by ustekinumab (2.2%), guselkumab (2.5%), secukinumab (3.2%), adalimumab (3.4%), bimekizumab (two dosages pooled) (4.1%), and ixekizumab (4.3%). Consistent with these rankings, the SUCRA values were the highest for risankizumab (91.0%) and the lowest for ixekizumab (31.7%) and bimekizumab (two dosages pooled) (25.7%) (Table 1). No statistically significant differences were identified (Table 3).
Benefit–Risk Profiles
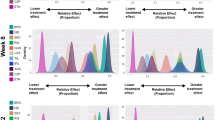
Risankizumab had the top SUCRA values for efficacy and safety outcomes, and thus had the most favorable benefit–risk profile (Fig. 3). Guselkumab also had relatively high rankings of both efficacy and safety outcomes. While bimekizumab and ixekizumab had favorable benefit profiles, the safety rankings of both treatments were among the lowest. Adalimumab, ustekinumab, and secukinumab had the lowest rankings of efficacy compared with the other treatments.
Long-term benefit–risk profiles for licensed and investigational treatments for moderate-to-severe plaque psoriasis. a SUCRA for PASI 75/90/100 responses vs. SUCRA for any AE. b SUCRA for PASI 75/90/100 responses vs. SUCRA for any SAE. c SUCRA for PASI 75/90/100 responses vs. SUCRA for AEs leading to treatment discontinuation. ADA Adalimumab, AE adverse event, BKZ bimekizumab, GUS guselkumab, IXE ixekizumab, PASI Psoriasis Area and Severity Index, PASI 75, 90, 100 at least a 75, 90, or 100% decrease from baseline PASI score, RIS risankizumab, SEC secukinumab, SUCRA surface under the cumulative ranking curves, UST ustekinumab
Discussion
This study utilized up-to-date clinical trial data to indirectly compare the long-term efficacy and safety of licensed and investigational therapies for moderate-to-severe plaque psoriasis and characterize their relative benefit–risk profiles. In the NMA of long-term efficacy, risankizumab and bimekizumab were associated with the top PASI response rates and relative rankings. Risankizumab was also associated with the lowest rates and the highest relative rankings for any AE, any SAE, and AEs leading to treatment discontinuation in the NMA of long-term safety. These data suggest that risankizumab has the most favorable long-term benefit–risk profile compared with the other treatments for moderate-to-severe psoriasis.
While there have been many NMAs characterizing the short-term efficacy and safety of treatments for moderate-to-severe plaque psoriasis [12, 17, 20, 21, 25,26,27,28,29,30,31,32,33,34], only a few studies have examined those in the long term [17, 20, 21, 35, 36]. The results of the current long-term efficacy and safety NMAs, including PASI response rates and safety event rates, are consistent with the findings of previously published long-term NMAs and meta-analyses of licensed treatments for psoriasis. Specifically, prior studies have demonstrated that risankizumab was associated with the highest PASI response rate and the lowest safety event rates [17, 20, 21, 35]. In addition, an NMA by Blauvelt et al. [36], comparing cumulative days at PASI 90 and 100 for biologic treatments for psoriasis, found that the cumulative days at PASI 90 and 100 were the highest for risankizumab and ixekizumab, respectively. Furthermore, the long-term benefit–risk profiles characterized in the present study are similar to the ones reported by Shear et al. [17], with risankizumab being the most favorable. The comparative long-term benefit–risk profiles in the present study based on clinical trial data are also similar to the ones reported by Torres et al. [18] using drug survival as the metric, although that real-world study did not include investigational therapies.
Compared with the previously published long-term NMAs which focused on licensed treatments [17], the present study included bimekizumab, an investigational treatment with safety and efficacy data available in the publications from several phase 3 clinical trials [7,8,9,10], as one of the comparators. The PASI response rate for bimekizumab was among the highest, which was significantly higher than multiple previously licensed treatments for psoriasis, including ixekizumab. Compared with the other treatments in the NMA, bimekizumab had the lowest ranking for any AE and AEs leading to treatment discontinuation. The NMA found that the rate of any AE for bimekizumab was significantly higher than the rates for the two anti-IL-23 agents (risankizumab and guselkumab). Additionally, the BE RADIANT trial of bimekizumab versus secukinumab reported that 21.2% and 4.6% of patients in those respective arms experienced Candida infections during the period from weeks 0 to 48 [10]. Conversely, the 52-week IMMERGE trial of risankizumab versus secukinumab reported that seven of 327 patients in total experienced Candida infection (until 20 weeks after the last dose of study drug administration) and that the infections were evenly distributed between the two arms [37]. Sbidian et al. [12] also conducted a risk–benefit analysis of treatments for moderate-to-severe psoriasis, including bimekizumab, and suggested that risankizumab and bimekizumab had favorable short-term benefit–risk profiles based on PASI 90 response rates and SAEs. However, the results for bimekizumab were considered to be of low certainty as the phase 3 trial data were not available for inclusion at the time of the study’s conduct.
This study is subject to several limitations, some of which are inherent to indirect comparisons. First, as with other NMAs, potential cross-trial differences in trial characteristics, patient characteristics, and outcome assessment (e.g., time points, AE assessment and collection) may undermine the comparability of outcomes across trials. Second, given the sparsity of the networks, it was infeasible to implement a random-effects or meta-regression model to capture or explain the potential heterogeneities in contrasts between treatments connected by more than one trial (e.g., risankizumab vs. ustekinumab). Third, as the study synthesized clinical trial data, the results may not necessarily be generalizable to all patients with moderate-to-severe plaque psoriasis in the real world.
As more long-term data are becoming available for treatments for moderate-to-severe plaque psoriasis, future studies may leverage a richer set of trial data to understand the impact of cross-trial heterogeneities in patient and trial characteristics on the comparative long-term benefit–risk profiles. With a richer network, techniques such as random-effects NMA models and traditional and multilevel network meta-regression [38] could be considered. Additionally, validating the long-term benefit–risk profiles of the therapies considered in this study in the real world will also augment the present results.
Conclusions
Risankizumab and bimekizumab had the highest long-term PASI response rates, and risankizumab had the lowest long-term rates of safety events. Therefore, risankizumab was associated with the most favorable long-term benefit–risk profile. While bimekizumab and ixekizumab had favorable efficacy profiles, both treatments had higher rates of safety events/outcomes and lower rankings for safety outcomes compared with risankizumab.
References
AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis—comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59(5):566–71.
Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–6.
Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
Sampogna F, Gisondi P, Melchi CF, et al. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol. 2004;151(3):594–9.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Honma M, Hayashi K. Psoriasis: recent progress in molecular-targeted therapies. J Dermatol. 2021;48(6):761–77.
Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98.
Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–86.
Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–41.
Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52.
European Medicines Agency. Product information: Bimzlex (bimekizumab). https://www.ema.europa.eu/en/medicines/human/EPAR/bimzelx#product-information-section. Accessed 2 Nov 2021.
Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1(1):Cd011535.
Mrowietz U. Implementing treatment goals for successful long-term management of psoriasis. J Eur Acad Dermatol Venereol. 2012;26(suppl_2):12–20.
Boehncke WH, Boehncke S, Tobin AM, Kirby B. The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20(4):303–7.
Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol. 2017;76(3):393–403.
Sain N, Willems D, Charokopou M, Hiligsmann M. The importance of understanding patient and physician preferences for psoriasis treatment characteristics: a systematic review of discrete-choice experiments. Curr Med Res Opin. 2020;36(8):1257–75.
Shear NH, Betts KA, Soliman AM, et al. Comparative safety and benefit–risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. J Am Acad Dermatol. 2021;85(3):572–81.
Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–79.
Mourad AI, Gniadecki R. Biologic drug survival in psoriasis: a systematic review & comparative meta-analysis. Front Med. 2021;7:625755.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther. 2021;11(3):885–905.
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17.
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58.
Mahil SK, Ezejimofor MC, Exton LS, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. 2020;183(4):638–49.
Signorovitch JE, Betts KA, Yan YS, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol. 2015;172(2):504–12.
Armstrong AW, Betts KA, Signorovitch JE, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018;34(7):1325–33.
Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS ONE. 2019;14(8): e0220868.
Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;10:2546161.
Xu G, Xia M, Jiang C, et al. Comparative efficacy and safety of thirteen biologic therapies for patients with moderate or severe psoriasis: a network meta-analysis. J Pharmacol Sci. 2019;139(4):289–303.
Xue W, Saharia P, Gray E, et al. Efficacy of brodalumab for moderate to severe plaque psoriasis: a Canadian network meta-analysis. J Cutan Med Surg. 2020;24(6):561–72.
Tada Y, Watanabe R, Noma H, Kanai Y, Nomura T, Kaneko K. Short-term effectiveness of biologics in patients with moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. J Dermatol Sci. 2020;99(1):53–61.
Warren RB, Gooderham M, Burge R, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2020;82(5):1138–49.
Sawyer L, Fotheringham I, Wright E, Yasmeen N, Gibbons C, Holmen MA. The comparative efficacy of brodalumab in patients with moderate-to-severe psoriasis: a systematic literature review and network meta-analysis. J Dermatol Treat. 2018;29(6):557–68.
Yasmeen N, Sawyer LM, Malottki K, Levin L, Didriksen Apol E, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: A systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2020. https://doi.org/10.1080/09546634.2020.1743811.
Blauvelt A, Gooderham M, Griffith CE, et al. Cumulative clinical benefits of biologic treatments for psoriasis over 1 year. Presented at American Academy of Dermatology Virtual Meeting Experience (AAD VMX) on 23–25 April, 2021 (virtual).
Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase 3, randomised, open-label, efficacy assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–9.
Phillippo DM, Dias S, Ades AE, et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J R Stat Soc Ser A Stat Soc. 2020;183(3):1189–210.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28.
Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): Results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–9.
Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17.
Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60-9.e9.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Bagel J, Blauvelt A, Nia J, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol. 2021;35(1):135–42.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70-9.e3.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–61.
Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–9.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by AbbVie Inc.
Medical Writing
Editorial assistance in the preparation of this article was provided by Dr. Shelley Batts of Analysis Group, Inc. Support for this assistance was funded by AbbVie Inc.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Yan Wang and Yawen Gao. The first draft of the manuscript was written by Yan Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
Portions of this research are planned to be presented at the 9th International Congress, Psoriasis: from Gene to Clinic, occurring during 9–11 December, 2021, and the 2022 American Academy of Dermatology (AAD) Annual Meeting, occurring during 25–29 March, 2022 in Boston, MA, USA.
Disclosures
April W. Armstrong serves as investigator and/or scientific advisor to AbbVie, BMS, Incyte, Leo, UCB, Janssen, Lilly, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed. Ahmed M. Soliman and Vassilis Stakias are employees of AbbVie and own AbbVie stock. Luis Puig has served as investigator and/or consultant or paid speaker to AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Janssen, Leo, Lilly, Merck, Novartis, Pfizer, and UCB. Keith A. Betts, Yan Wang, and Yawen Gao are employed by Analysis Group, Inc., which received payment from AbbVie Inc. for participation in this research.
Compliance with Ethics Guidelines
As this is a post-hoc NMA of previously published results of clinical trial data, no institutional board review was required. The study was performed in accordance with the Helsinki Declaration 1964 and its later amendments. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are in the electronic supplementary materials of the published article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Armstrong, A.W., Soliman, A.M., Betts, K.A. et al. Long-Term Benefit–Risk Profiles of Treatments for Moderate-to-Severe Plaque Psoriasis: A Network Meta-Analysis. Dermatol Ther (Heidelb) 12, 167–184 (2022). https://doi.org/10.1007/s13555-021-00647-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00647-0