Abstract
The differential mobility analyzer (DMA) is a narrow-band linear ion mobility filter operating at atmospheric pressure. It combines in series with a quadrupole mass spectrometer (Q-MS) for mobility/mass analysis, greatly reducing chemical noise in selected ion monitoring. However, the large flow rate of drift gas (~1000 L/min) required by DMAs complicates the achievement of high gas purity. Additionally, the symmetry of the drying counterflow gas at the interface of many commercial MS instruments, is degraded by the lateral motion of the drift gas at the DMA entrance slit. As a result, DMA mobility peaks often exhibit tails due to the attachment of impurity vapors, either (1) to the reagent ion within the separation cell, or (2) to the analyte of interest in the ionization region. In order to greatly increase the noise-suppression capacity of the DMA, we describe various vapor-removal schemes and measure the resulting increase in the tailing ratio, (TR = signal at the peak maximum over signal two half-widths away from this maximum). Here we develop a low-outgassing DMA circuit connected to a mass spectrometer, and test it with three ionization sources (APCI, Desolvating-nano ESI, and Desolvating low flow SESI). While prior TR values were in the range 100–1000, the three new sources achieve TR ~ 105. The SESI source has been optimized for maximum sensitivity, delivering an unprecedented gain for TNT of 190 counts/fg, equivalent to an ionization efficiency of one out of 140 neutral molecules.

ᅟ
Similar content being viewed by others
Introduction
In spite of the much larger resolving power of other mass analyzers, the quadrupole mass spectrometer (Q-MS) remains one of the most widely used types, thanks to its ability to operate as a narrow-band mass filter. Indeed, extracting a single ion mass out of a complex mix for subsequent study or manipulation is key in many scientific and analytical situations. An ion mobility spectrometer (IMS) capable of continuously drawing a narrow range of ion mobilities out of a complex mixture is equally useful, with the added advantage of performing its function in a gas that may be at atmospheric pressure. This filter type permits, for instance, studying the kinetics of ion [1] or neutral [2] evaporation, carrying out spectroscopy in mobility-selected species, etc. The combination in series of such a mobility filter with a Q-MS for single ion monitoring (SIM) offers transmission efficiencies and analysis times similar to those required for Q-MS analysis, but with greatly increased resolving power [3].
Several narrow-band ion mobility filters operating in a steady or quasi-steady form are known. Field asymmetric IMS (FAIMS), also referred to as differential mobility spectrometry (DMS), combines a strong AC and a weak DC field [4]. Its original poor resolution has been considerably alleviated by the choice of a drift gas including either polar vapors, such as 2-propanol [5], or light components, such as He and H2 [6]. FAIMS separation depends on nonlinearities of the drift velocity on the electric field, which are difficult to interpret in terms of cross-section. Transversal modulation IMS (TMIMS) combines a DC axial field with an orthogonal AC field of comparable magnitude, to continuously focus on an exit slit only those ions whose (mobility-dependent) axial time of flight (TOF) resonates with the imposed period of transverse oscillation [7]. TMIMS operates in the linear mobility regime, hence providing cross-section information. In addition to the main resonance, TMIMS also passes higher harmonics, and leaks a small amount of other mobilities in the form of pulses at the imposed transverse frequency. These problems can, however, be overcome, for instance with two TMIMS filters in series [8]. Other promising proposals are the periodic focusing differential mobility analyzer (PFDMA) [9], where ions of a selected mobility are purified by selective passage over numerous slits in the presence of electric and flow fields, and overtone mobility spectrometry [10] that allows a linear drift tube to be operated as a continuous ion mobility filter.
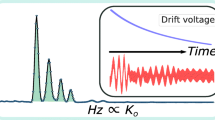
Here, we focus on the differential mobility analyzer (DMA) [11], a member of a class of narrow-band ion mobility filters combining a DC electric field and a steady flow field of drift gas [12], and providing linear mobility separation. The DMA operates at ambient pressure, and can be easily added or removed from the ion source region of atmospheric pressure ionization (API) MS systems [13], a combination (DMA-MS) having demonstrated excellent performance in a variety of analytical and basic applications [14–17]. From the analytical perspective, the DMA can be viewed as a chromatography of sorts, removing 98%–99% of the ion mobilities in a complex mix [18], while being able to shift from one mobility to another in a few milliseconds. This noise-suppression ability has been demonstrated in monitoring for explosives in the exceedingly complex mixture of vapors characteristic of the atmosphere at concentrations as low as 0.01 parts per quadrillion (1 ppq = 10–15 atmospheres of partial pressure) [19]. At this sensitivity, a limiting feature as important as resolution [defined as the mean ion mobility divided by the full mobility width at half maximum (FWHM)] is the presence of small tails (often associated with incomplete desolvation of abundant contaminant species), which may completely obscure the presence of traces of analytes of interest. This tailing is illustrated in the mobility spectrum of Figure 1c, where the DMA voltage, shown as horizontal variable, is inversely proportional to ion mobility. One sees substantial background in the voltage range 2200 > VDMA (Volt) > 1070, where any small peaks present would be undetectable. In order to quantify these tails, we define the tailing ratio TR as the signal at the peak maximum divided by the signal two half-widths away from this maximum (TR; Figure 1a). For a Gaussian peak (characteristic of diffusion broadening) TR = 216 ~ 0.655 × 105, yet TR values found in Figure 1c and in prior DMA-MS studies are rather in the range 102–103.
Illustration of the Tailing Ratio TR in several settings: (a) Definition of TR. (b) Normalized nitroglycerin peak in a high TR DMA configuration (TR ~ 105) using Desolvating-nanoESI as ionization source. (c) Normalized nitroglycerin peak in a DMA configuration with a low TR of 3.2 × 102, using LFSESI [20] as ionization source
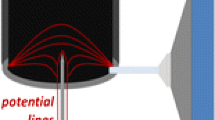
No systematic study is available on such tailing phenomena in DMAs, but two characteristic features of this instrument suggest that it would be more prone to peak tailing than other IMS systems: First, DMAs of high resolving power use large flows of drift gas (~1000 L/min), complicating the achievement of gas dryness levels common in drift-time IMS. Additionally, conventional curtain [21, 22] or counterflow [23] heated drying gas schemes used in API-MS to avoid vapor ingestion owe much of their success to the coincidence of the symmetry axes of the drying gas flow and the inlet orifice to the MS. This symmetry and the corresponding high drying efficiency, however, is broken at the sample inlet slit of the DMA, due to the motion of the DMA drift gas orthogonally to this axis (Figure 2). Accordingly, our main goal here is to develop more effective vapor-suppression schemes for DMAs, so as to minimize peak tailing, to reach TR values close to those for an ideal Gaussian peak (0.655 × 105).
Several typical tailing phenomena arising in DMAs are illustrated in Figure 1c, which is a mass-selected mobility spectrum for nitroglycerin (NG), where the DMA is followed by a triple quadrupole transmitting the 262/46 ion, with parent at 262 m/z (NG + Cl–) and its NO2 – fragment at 46 m/z. The DMA voltage variable shown in the mobility spectrum is inversely proportional to ion mobility (hence high and low mobility tails are, respectively, to the left and to the right of the peak). The ions were formed by electrospraying a solution of dimethyl sulfoxide:octanol 75:25 in volume, HCl 0.1% + nitroglycerin (NG) 5 ppmv. This high boiling point solvent maintains a stable electrospray at 200 °C, but complicates an effective desolvation of the ES cloud. For this reason, it has been replaced by methanol/water (9/1) in all the electrospray ionization sources subsequently used in this study.
The peak of the dominant ionizing species Cl– (not shown in the figure), appears at V DMA = 1070 V, coinciding with the beginning of the high mobility tail of the NG-Cl- peak. This creates a uniform background intensity ~10–5 invading the space between the voltages corresponding to the peaks of pure Cl– (V Cl = 1070 V) and pure NG-Cl- (V NG ~ 1700 V). This continuous signal between V Cl and V NG is due to attachment within the DMA of a neutral NG molecule to the Cl–. This results in a mobility spanning all intermediate values between the mobilities of Cl– and NG-Cl–. The presence of a neutral analyte vapor inside the DMA is explained by the imperfect DMA counterflow drying (Figure 2), which allows small quantities of neutral vapors to reach the DMA. This long left-tail structure is not unique to NG and Cl–, but arises similarly with many other analyte mobility peaks and ionizing species.
The low mobility tail of the NG-Cl– peak shown in Figure 1c extends from VDMA 1750 V to 2200 V (the end of the registered data), with signal intensity considerably higher than the high mobility tail. Low mobility tails are most likely due to various states of solvation of NG-Cl– ions formed prior to their entrance into the DMA and, to a lesser extent, due to the attachment within the DMA of some contaminant vapors to a specific ion formed upstream of the DMA.
Both high and low mobility tails are evidently undesirable. Both result from the presence of neutral vapors, and both would be removed with a thorough elimination of condensable species from the whole DMA circuit. These vapors have several possible origins. As in conventional IMS, they may be due (1) to an insufficiently purified source gas, (2) inadequate desolvation at the interface between the DMA and the ES source, and (3) internal outgassing within the instrument. The last source is harder to control in DMAs than in drift-time IMS because of the need for a blower to recirculate gas flow rates of the order of 1000 L/min. Particularly critical is the possibility of leakage of vapors from lubrication fluids penetrating into the recirculating DMA gas flow through the shaft powering the compressor blades.
Here we describe three new ionization chambers that significantly reduce solvent vapors at the interface with the DMA, as well as a low outgassing closed DMA circuit, the conduits of which are kept at high temperature except at the blower. The blower is preceded by an intercooler that maintains it near room temperature, limiting its vapor emissions.
Experimental
MS
Either AB Sciex’s QTRAP 5500 or AB Sciex’s TripleQuad 5500 were both operated as triple quadrupoles.
DMA
We used SEADM’s DMA P5-f, with critical dimensions collected in Table 1, which includes improvements over a similar instrument previously described [13]. An important practical innovation is that the flow-limiting orifice is no longer at the outlet slit of the DMA, but in the MS. The DMA can then be quickly removed or installed without breaking the MS vacuum. The DMA combines a horizontal laminar flow of gas with a vertical electric field between two parallel plates, such that ions of different mobilities penetrating through a slit in the upper plate open up in a fan as they drift towards the other plate, whereby only a small range of mobilities is sampled through a second slit in the lower plate (Figure 2) and transmitted to the MS. The materials used in the DMA are stainless steel (SS) AISI316 for the inlet/outlet electrodes and the drift gas recirculation circuit, aluminum for the DMA entrance and outlet diffuser parts, PEEK for the DMA insulator box, and Teflon encapsulated o-rings for the sealing between the different parts. The DMA-MS interface is made of SS AISI316, PEEK, and Teflon encapsulated O-rings. The DMA was operated at 120 C, thanks to a heater (aluminum) located between the DMA blower and the DMA.
Pump
The sealed DMA blower (Domel d.o.o., 791 series brushless blower) is made of aluminum and SS, with the exception of the shaft seal (unknown plastic). Ideally, this shaft should avoid the entrance of vapors emitted by the lubricated bearing located right beside it. However, as demonstrated later, when the blower runs at a high temperature (70 °C), vapors are released into the DMA’s drift gas, most likely from the lubricant and the plastic shaft seal. To further minimize these vapor emissions, an air intercooler (aluminum) placed between the blower inlet and the DMA outlet could cool the blower below 35 °C.
Analyte Vapor Ion Generation
Controlled amounts of sample vapor were introduced in the ionizer by heating a solid sample in a desorber consisting of an SS AISI 316 Swagelok T embedded within an aluminium heating block, whose temperature could be accurately set by a PID control. The solid samples to be desorbed are introduced into a SS AISI316 mesh that is inserted into the T. In order to minimize outgassing, use of plastic materials has been avoided also in the desorber and the transfer line. The solid source of vapor was a pill containing NG, commonly used for heart disease (Parke-David S.L., Vernies 0.4 mg Nitroglycerin). In order to have an adequate signal to noise ratio, we selected a desorber temperature yielding 106 cps of NG at the maximum of the mobility peak.
For some experiments involving an electrospray, 500 ppb of NG (Isostandards M-8330-ADD-1) is added to the electrospray solvent, which produces NG ions directly from the liquid phase. This approach injected into the system a minute NG flux of 3.6 pg/s. These vapor concentrations are large enough for the peak height to exceed 105 times the minimum detectable signal, a necessary condition to be able to measure TR ~105.
DMA-MS
Using the APCI source (Figure 3a) described in a later section, two-dimensional DMA-MS scans (Figure 4) were generated by sweeping the DMA voltage between 0 and –2500 V at 10 V steps. At each voltage step, a m/z Q1 sweep of 15–800 Da in negative mode at 1000 Da/s was carried out, with the experiment lasting 3.3 min in total. The aim of these experiments was not to infer TR, but to diagnose the content of vapors in the DMA circuit (see the Results section).
(a) APCI source + DMA. (b) Desolvating-nanoESI + DMA: the laminar flow of heated drying gas enters laterally through a metal frit and is symmetrically directed upwards, efficiently desolvating the ES drops, and preventing neutral vapors from entering the DMA. (c) Desolvating-LFSESI + DMA, where hot dry gas rises vertically through electrode 16 to meet frontally the electrospray cloud
Mobility-MS spectra for vapor background in the APCI source. (a) N2 from a generator (99.5% purity) with DMA/ion source temperature of 40 °C. (b) After running the system for 90 min with bottled N2 (99.9992% purity), with DMA and ion source temperature of 40 °C. (c) bottled N2 (99.9992% purity), with DMA at 120 °C (DMA heater present), DMA blower actively cooled to 35 °C, ion source at 230 °C. (d) DMA mobility spectra summing over all masses for experiments a, b, and c
TR Measurement
Ion mobility peaks are obtained by scanning the voltage difference applied between the two DMA plates, with the triple quadrupole mass spectrometer operating in multiple reaction monitoring mode, selecting the nitroglycerin precursor ion in the first quadrupole, and the product ion in the third quadrupole. Table 2 details the precursor and product ions depending on the ionization source used.
Measuring TR ~105 is not straightforward because the MS needs to have a dynamic range exceeding this high value. When no analyte is introduced, the NG MS/MS channel is practically free of background, while the MS detector saturates at ~5 × 106 counts/s. We therefore used a dwell time of 1 s and could quantify a minimum MS signal of one count per second (cps) and signals of about 106 cps. The dynamic range of the MS under those conditions was accordingly ~106, not limiting the measurement of TR.
APCI Source
A leading cause of solvation in the DMA is the penetration of ES solvent from the ionization region into the DMA. This external vapor source was eliminated to evaluate the influence on the TR of background from either the N2 gas used, or outgassing from either the DMA, the recirculation circuit or the blower. A discharge ionization source (APCI) entirely free from ES vapors was accordingly developed (Figure 3a). This chamber, due to its plastic-free construction, has a low level of outgassing. Its body is made of AISI316 stainless steel (SS). The gas inlet and outlet, like the 1/16″ tungsten electrode discharge inlet, is achieved through three Swagelok fittings welded to the chamber. The electric insulation between the tungsten discharge electrode and the chamber body (equipotential with the DMA inlet electrode) is achieved by using a ceramic insulator (Macor, Corning Inc., New York, USA) (Figure 3a- item 2). Aluminum flat gaskets are used to seal the various parts of the chamber, including unions between metallic and ceramic parts (Figure 3a- item 3). The metallic parts have sharp edges (Figure 3a- item 4) that create plastic deformation in the aluminium gaskets and a perfect seal between the parts. It is not possible to put sharp edges in the ceramic insulator because the high compressive stress created would break them, but because of the good surface finish of the ceramic insulator and the aluminum gaskets, the pressure created in the seat plane seals also the contact between the ceramic and the metallic gasket. No bubbles were observed when the assembly was pressurized at 1 bar with N2 and immersed in methanol. The gas used in the DMA drift gas circuit and the sample flow through the desorber was 99.9992% pure bottled nitrogen. The sample and counterflow flow-rates were 0.3 and 0.4 l/min, respectively. In order to compensate for the MS consumption of 2.5 L/min through its sampling orifice, 2.9 L/min of nitrogen were introduced into the recirculating DMA drift gas (2.5 L/min for the MS and 0.4 L/min for the counterflow). The N2 supply lines were of Teflon and stainless steel. The fittings (Swagelok) were made of SS. The valves were made of SS with PFA (Teflon) or PEEK seats, and the flowmeters used were SMC PFM710, wherein the parts in contact with the fluid were made of liquid crystal polymer (LCP), polybutylene terephthalate (PBT), brass (electroless nickel plated), nitrile butadiene rubber + fluoro coated, viton + fluoro coated, Au, stainless steel 304.
A cartridge heater connected to a PID control system allows the temperature of the ion source to be selected in the range of 40–350 °C. The ionization chamber is connected to the desorber by means of heated fused silica-lined stainless steel tubing.
Desolvating-NanoESI Ionization Source
The study with the APCI ionization source revealed that high DMA TRs were possible in the low outgassing DMA configuration, as long as no ES solvent was introduced. The next challenge was to achieve comparable TRs with an electrospray source. A custom nano-ESI source was designed specifically to achieve complete removal of vapors. It provides a heated drying gas flow upstream of the DMA inlet slit, symmetrically directed towards the electrospray cloud (Figure 3b). In this case the analyte ions were electrosprayed from the electrospray solvent (MeOH-H2O 9:1/HCl 0.1%) containing 500 ppb of NG.
In prior DMA-ES source interfaces, the drying gas originates from inside the DMA (Figure 2), where it moves towards the left at speeds of many tens of m/s. Therefore, the drying gas jet emerging outwards through the slit impacts the ES cloud at a substantial angle, being unable to fully avoid diffusion of vapors from the spray into the DMA. In the present design, 1.5–3 L/min of drying gas are symmetrically injected through a metallic frit (Figure 3b- item 12) into the spray region, opposing the spray cloud head on (Figure 3b- item 5). In order to further reduce penetration through the slit of DMA gas carrying a leftward velocity, it is possible to inject a small amount of drying gas into the DMA. In practice, the drying performance is not found to be impaired even if this flow through the slit is slightly reversed (–0.2 L/min). The temperature of the drying gas is adjustable between 70 °C and 220 °C via heaters acting upstream from the metallic frit and in the frit itself.
The internal source geometry through which the drying gas desolvates the sample ions includes an initially lateral entry through the frit, followed by a 90° upward turn, with a slightly converging region 13 mm long, which accelerates the gas to avoid turbulent transition. The fluid velocities in the electrospray region were 3 and 6 m/s for flow rates of 1.5 and 3 l/min, with corresponding Reynolds numbers Re = 180 and 360 (based on a lateral characteristic length of 1.6 mm and a kinematic viscosity ν = 2.5 10–5 m2/s for nitrogen at 120 °C). These moderate Re values suffice to preclude diffusion of neutral vapors and incompletely dried drops from the ionization chamber into the DMA slit.
In order to compensate for the MS consumption of 2.5 L/min through its sampling orifice, the same flow of nitrogen was introduced into the recirculating DMA drift gas. The electrospray silica capillary used was a New Objective (Woburn, Massachusetts, USA) TaperTip: 360 μm o.d., 50 μm i.d., 50 cm in length, noncoated (ref. TT360-50-50-N-5).
The gas used in the DMA drift gas circuit and in the heated drying gas was produced by a nitrogen generator (NitroFlow basic from Parker Hannifin (Cleveland, USA) 99.95% purity). This nitrogen is subsequently purified using oxygen and hydrocarbon traps (SGT Oxigen TrapCO1002 and Hydrocarbon Trap CO1003). With this nitrogen configuration, the same DMA TR is found as when using 99.9992% purity bottled nitrogen, indicating that extremely high nitrogen purity is not essential to achieve good DMA TR.
The nozzle guiding the drying gas is metallic and floats 1.8 kV above the DMA inlet electrode, yielding a local electric field of ~1 kV/mm. The electrospray tip floats above the metallic nozzle at a voltage of 1.2 kV. With this electric configuration, the residence time of an ion with mobility of 1 cm2/V/s, from the electrospray to the DMA (separated 3 mm), is approximately 37.5 μs, resulting in negligible dilution effects by space charge and diffusion.
The DMA, the drift gas recirculation circuit, and the DMA blower were the same ones used with the APCI source. The intercooler and DMA heater were used at all times. Only Teflon tubing was used in the N2 supply lines.
Desolvating-LFSESI Ionization Source
After successful development of the Desolvating-nanoESI ionization source, a secondary ESI (SESI) source was designed for vapor ionization. This SESI source differs from the prior ESI source in that the drying gas cannot be allowed to flow through the ES region. Otherwise it would dilute the incoming sample vapor, whose flow rate needs to be very small in order for the sample to be efficienly ionized [20]. An intermediate “impact” chamber (Figure 3c- region between electrodes 15 and 16) must therefore be introduced, where the small sample flow collides frontally against the drying gas, both streams being rejected laterally, while the ionized species are driven downstream by the field [20]. Evaluation of TR for the SESI setup relied as before on NG ions introduced by electrospraying a 500 ppb NG solution. This convenient ion source was suitable to test the system’s ability to remove vapors, but could not measure the SESI ionization efficiency. For evaluation of the ionization efficiency the NG was withdrawn from the ES solvent, and small quantities (pg) of a solution containing various explosives was deposited in a filter. This material was subsequently vaporized in a thermal desorber into the sample gas fed to the ionizer. A relatively volatile electrospray solution MeOH-H2O 9:1, HCl 0.05% was used, which tends to boil at the high temperature (170 °C) required to prevent internal contamination in the ionization source (analyte memory effects). The lack of an electrospray cooling casing had limited the viable range of liquid flow rates and temperatures in the prior ESI source (Figure 3b), so this feature (Figure 3c- item 13) was added here to protect the liquid meniscus from the heated first electrode facing it (Figure 3c- item 14).
An electric field between the electrospray liquid and a first electrode (Figure 3c- item 14) (1.5 kV/mm) drives the ions and droplets generated by the electrospray towards the vapor ionization region, defined by the first and second electrodes (Figure 3c- items 14 and 15, respectively). A 0.2 lpm sample flow carries the explosive vapors from the desorber to the vapor ionization region through eight holes that create an approximately symmetric flow (Figure 3c- sample flow lines). In the ionization region, analyte vapor molecules interact with the electrospray ions and droplets, generating analyte ions that drift towards the next region through an electric field (0.3–0.08 kV/mm) between the first and second electrodes. The magnitude of this electric field modifies the trajectories of the field lines in the ionization region. The corresponding change in ion residence time has a strong influence on the efficiency of vapor ionization, which should ideally be optimized for each analyte. High electric fields improve the gain (counts/femtogram) for chemically labile analytes, while low electric fields improve the gain for stable and temperature-resistant analytes.
The impact region defined between the second and third electrodes (Figure 3c- items 15 and 16, respectively) prevents the drying gas counterflow (Figure 3c- black filled lines) from diluting the sample vapors, avoiding associated reductions in the efficiency of ionization [20]. Large droplets and neutral vapors from non-ionized sample and from the electrospray solvent are eliminated through this impact region. The electric field between the second and third electrodes (0.4 kV/mm), pushes the ions towards the next desolvation region, delimited by the third electrode and the DMA inlet electrode (Figure 3c- items 16 and 6, respectively). Small charged droplets are completely desolvated in this region via a symmetric heated drying gas counterflow N2 current (Figure 3c- black filled lines). This stream (1–3 L/min) desolvates small droplets, dragging upwards the neutral vapors produced by the evaporation process to the impact region and later to the exhaust outlet. An electric field (0.5 kV/mm) between the third electrode and the DMA inlet electrode drives the ions downwards against the drying counterflow current, injecting them into the DMA. The drying gas reaches the temperature of the third electrode while transiting through a labyrinthine path milled in this part. The temperature in each electrode is independently adjustable, though the temperature used in this study was 170 °C in all three electrodes. Small flows (~0.25 lpm) through the DMA entrance slit in both directions do not affect the desolvation efficiency.
The DMA outlet electrode (Figure 3c- item 7) is at the same potential as the MS orifice (approximately grounded), whereas the inlet electrode (Figure 3c- item 6) floats above it; the high voltage power supply (HVPS) used to ramp the DMA inlet electrode voltage was an applied kilovolts HP-005ZIP025. The HVPSs used in the three electrodes of the Desolvating-LFSESI and in the electrospray (Figure 3c- items 16, 15, 14, and 11) were EMCO E-series. In the Desolvating-nanoESI two EMCO E-series were used for the nozzle electrode and for the electrospray, and in the APCI only one EMCO E-series was used for the corona discharge.
At a DMA blower speed of 12000 revs/min, all the explosives left the DMA at voltages lower than –1700 V. Table 3 shows the voltages used in the different electrodes and electrospray in the test reported in this work, as well as in the optimization tests.
The EMCO HVPS, especially the electrospray HVPS, which is at the highest voltage (it floats above the other voltages), withstood the voltages reported with no failure.
The electrospray silica capillary used was a New Objective TaperTip: 360 μm o.d., 50 μm i.d., 15 μm tip i.d., 80 cm in length, noncoated (ref. FS360-50-15-N-5-C80). The desorber is made of aluminum with Viton gaskets, and the transfer line that joins the desorber with the ionization chamber consists of heated fused silica-lined stainless steel tubing. The N2 used, the DMA, the drift gas recirculation circuit, the DMA blower, and the N2 supply lines were as in the Desolvating-nanoESI source. The DMA blower intercooler and the DMA heater were used at all times.
For the gain calculations presented in Table 4, 2 μL of explosive dissolved in MeOH are deposited into a stainless steel mesh filter + Tenax GR + glass fiber. The concentration of the explosive in the solvent is 50 ppb for EGDN, 12.5 ppb for NG, and 2.5 ppb for RDX, PETN, and TNT. After the solvent evaporates, the filter is inserted into the desorber, where the solid sample vaporizes at a temperature of 200 °C, the same temperature as the transfer line that carries the sample vapors to the ionization chamber. The DMA is synchronized with the MS (TripleQuad 5500), and selects an explosive every 100 ms, which the MS measures in multiple reaction monitoring mode. The DMA voltage is calibrated periodically by an internal calibrant present in the ES solvent. Extrapolation allows the voltage V DMA for each explosive’s peak to be determined, as this value varies with changes in atmospheric pressure. The extrapolation coefficients were calculated prior to the experiment.
The gain factor is obtained by integrating, for 45 s, the counts per second registered in the desorption spectrum, omitting the background present before inserting the filter into the desorber.
Results
APCI Source
As previously noted, the lack of ES solvent in this ionization source permits studying the effect of N2 purity and DMA blower temperature, on the internal background and on the TR of the DMA.
Figure 4 includes several two-dimensional (2D) DMA-MS spectra with m/z for the first quadrupole Q1 (no fragmentation in Q2, with Q3 transmitting all ions) in the vertical axis, the DMA voltage V DMA (inversely proportional to ion mobility) in the horizontal axis, and ion signal represented in a logarithmic color scale. Figure 4a (relatively impure gas from the N2 generator) shows numerous long horizontal lines, bound to the left at ~50 V (Zmax > 40 cm2/V/s), and to the right by a broad inclined band. Zmax is far too large to correspond to ions drifting in N2, so these long lines must originate from electrons entering into the DMA. These electrons attach to neutral vapors within the analyzer region, resulting in mobilities intermediate between their own (left bound of the line) and that of the ionized vapor (right bound of the line). Many electrons generated by the negative discharge evidently survive in the N2 environment of the ionizer, and then attach to electronegative species present dominantly in the closed DMA circuit. Electron attachment to vapor species within the DMA accordingly provides a sensitive diagnostic tool to evaluate the level of gas-phase contamination of the closed DMA circuit.
Figure 4a shows additional horizontal bands beginning at ~1050 V. This voltage corresponds to NO2 - and NO3 - ions (m/z 46 and 62), abundantly produced by the negative discharge. Like the electrons, these ions penetrate the DMA and ionize vapor impurities in the drift gas. Interestingly, the mass interval separating the most intense high-mobility tails is 50 Da, both for vapor ionization with electrons and ions; m/z = 50 corresponds to CF2, the repeat unit of Teflon [24, 25], probably from the compressor pistons of the N2 generator used. The striking observation that neutral (CF2)n fragments with masses going well beyond 700 m/z are volatile enough to produce such a considerable signal may be explained in part by the high electron ionization efficiency of electronegative species.
Comparing Figure 4a and b, one notices a significant (5-fold) reduction in internal background, achieved by substituting N2 from a generator (99.5% purity) with N2 from a bottle (99.9992% purity) (Figure 4d, circles and crosses). The marked reduction in the high mobility tails with 50 Da separation confirms that most of the (CF2)n impurities originate from the N2 generator. Indeed, when using the vapor traps on the line coming from the N2 generator, the high mobility tails decay substantially, but remain more intense than when using 99.9992% purity bottled N2.
In view of the high purity of the N2 used in Figure 4b, much of the remaining background must originate from the DMA. We substantially reduced vapor release from the DMA blower by refrigerating it down to 35 °C via an intercooler. The DMA and the rest of the drift gas recirculation circuit are maintained at 120 °C by a DMA heater, with the aim of minimizing surface adsorption of contaminants. We also heat up the ionization chamber, in this case up to 230 °C, leading to the “Low Outgassing DMA” spectrum shown in Figure 4c. Comparing scenarios (c) and (a), the counts received in the MS detector are reduced by a factor of 30 (Figure 4d, continuous line versus circles). The high mobility tail levels (between 0 and 600 V) quantifying vapor contamination within the DMA are reduced by a factor >200, but traces of them remain, perhaps from the Teflon-coated o-rings used in the DMA.
Figure 5 shows mass-resolved mobility spectra for nitroglycerin, with and without pump cooling. Both the heater and the intercooler were included in the DMA circuit. However, in the first experiment (line with triangles in Figure 5 left) the intercooler fan was not working, resulting in a DMA pump temperature of 83 °C with both high and low mobility tails in the spectrum that yielded TR = 1.5 × 104. Since we were working with a solvent-free source, acceptable TR were obtained even with a hot DMA blower. Forty min after the intercooler fan started working and the blower temperature reached 28 °C, both high and low mobility tails were strongly reduced, yielding TR ~105 (line with squares in Figure 5 left).
We have analyzed the TR of NG peaks in the Low Outgassing scenario of Figure 4c. In a sample of five mobility sweeps, we obtain an average TR of 1.7 × 105. The fact that it exceeds the ideal value for a Gaussian (0.655 × 105) suggests that the tails of our mobility peaks do not have a perfect Gaussian shape. Figure 5 right shows the shape of a NG peak obtained with the APCI source. Intensity points higher than 1 × 10–1 fit a Gaussian well, but the deeper tail of the peak decays faster, explaining the larger TR obtained.
Desolvating-NanoESI Ionization Source
At fixed electrospray conditions and electric fields, the two factors affecting desolvation most are the drying gas flow rate and its temperature. The heated drying gas effectively desolvates the ES droplets, limiting low mobility tails. The drying gas avoids vapor ingestion and DMA drift gas contamination responsible for low and high mobility tails. Figure 6a and b show the dependence of TR on drying gas flowrate and temperature (a), or on signal intensity (b). Higher drying gas counterflows achieve better desolvation for the same drying gas temperature, but the signal is far higher at the smaller gas counterflow. Working with a 160 °C drying gas temperature, the TR achieved is approximately the same in both drying flow rate scenarios, about TR = 1.3 × 105. Figure 6b shows a moderate loss of signal working with 1.5 L/min drying gas counterflow, due to thermal degradation of nitroglycerin when the temperature increases. This loss is not noticeable at 3 L/min drying gas counterflow.
The optimal operational conditions depend on the analyte, but it is generally preferable to operate with low drying gas flow rates (1.5 L/min) and as high as possible drying gas temperatures (160 °C), in order to reduce the residence time of the ions in the ionization chamber, in turn minimizing the loss of transmission associated to Brownian diffusion and space charge.
Desolvating-LFSESI Ionization Source
In view of the excellent performance of the Desolvating-nanoESI ionization source (with NG ions originating from the liquid phase), we proceeded to test the Desolvating-LFSESI designed to ionize neutral vapors in the gas phase. The parameters defining the operating point are drying gas flow, residence time (that depends on the sample flow rate from the desorber and on the electric fields in the ionization source), the temperature of each of the three ionization source electrodes, the pressure in the capillary of the electrospray, and its tip diameter. Figure 7 presents TR results obtained after optimizing these parameters, also comparing them with those of SEADM’s prior LFSESI, having no intercooler for the DMA blower and no DMA heater. The average enhancement factor of TR for SESI is 143. Table 4 compares the gain obtained with these two ionizers for various explosives, with MS/MS settings summarized in Table 5. These tests introduced the explosives in vapor form with the sample gas. The operational conditions for the comparison are summarized in Table 6. The mass spectrometers were the same. The DMAs changed slightly due to a modification in the depth of the entrance slit, which was reduced from 0.15 mm (P5-e DMA) to 0.093 mm (P5-f DMA).
The optimization of electric fields and other design parameters have increased the sensitivity by a factor of 3. These improvements include a smaller capillary tip (15 μm versus 50 μm, with a smaller tendency to arc in negative mode), and the shift to a methanol/water electrospray that dries much faster yielding ions closer to the tip. The depth reduction in the DMA entrance slit also contributed to the improvement of gain registered. Notice that a TNT ion is detected for each 1.4 104 neutral TNT molecules desorbed. Assuming that the MS transmission efficiency of ions from the atmospheric inlet to the detector is 10-2 in MS/MS mode, and taking the DMA to transmit 100% of the ions, one out of every 140 neutral TNT molecules would be ionized! This ionization probability may appear as small given that electrospray ionization is in some instances able to convert 100% of specific solution species into ions. Notice, however, that SESI (secondary ESI) ionizes gas-phase vapors rather than solutes, and does so far less efficiently by charge transfer from solution ions (Cl– in our case) into neutral vapors. Given enough time of exposure of the vapors to the spray, an equilibrium condition is reached where the rate of ion production is balanced by the rate of dilution by space charge. In this limit the estimated equilibrium ratio between ionized and neutral vapors for a unipolar charger is of the order of n i /n v ~ 10–4 [26]. The vapor ionization probability achieved of 1/140 is accordingly remarkably high. Much of the improvement observed is due to the previously described low flow SESI approach [20], based on which a gain of 30 counts/fg had been demonstrated previously. The ionization probability of LFSESI manages to exceed greatly the equilibrium n i /n v value because the ions are quickly extracted by the fields as they are produced, while the vapor is discarded very slowly by the prevalent low sample flow rate. Our measurements here with that earlier SESI source achieve a slightly higher 40 counts/fg, while the new Desolvating LFSESI source reaches 190 counts/fg, almost five times larger.
Conclusions
Three new ionization sources specially designed to be coupled to a DMA have achieved complete elimination of vapors and associated ion clustering. Combined with an essentially vapor-free DMA gas recirculation circuit (including a cooled commercial blower), this improvement yields ideal tail-free mobility peaks with a TR much larger than previously observed, reaching the theoretical limit ~105. This improvement has led to a greatly enhanced capacity of the DMA-MS combination to analyze complex mixtures, including explosives in the atmosphere at concentrations below 10–2 ppq [19]. The Desolvating-LFSESI source has been optimized for maximum sensitivity, delivering an unprecedented gain for TNT of 190 counts/fg, equivalent to an ionization efficiency of one out of 140 neutral molecules. Electron, Cl, NO2 and NO3 ionization within the DMA was used as a sensitive diagnostic tool to evaluate the level of gas-phase contamination of the closed DMA circuit. Extremely high nitrogen purity is not essential to achieve good DMA TR in a background free MS/MS channel, as comparable TR values are obtained with 99.9992% and 99.95% purity nitrogens.
References
Hogan, C.J., Fernandez de la Mora, J.: Ion-pair evaporation from ionic liquid clusters. J. Am. Soc. Mass Spectrom. 21, 1382–1386 (2010)
Hogan, C.J., Fernandez de la Mora, J.: Tandem ion mobility-mass spectrometry (IMS-MS) study of ion evaporation from ionic liquid-acetonitrile nanodrops. Phys. Chem. Chem. Phys. 11, 8079–8090 (2009)
Fernández de la Mora, J., Casado, A., Fernández de la Mora, G.: Method to accurately discriminate gas phase ions with several filtering devices in tandem. US patents 7,855,360 (December 21, 2010), 278,622 (2/October/2012)
Purves, R.W., Guevremont, R., Day, S., Pipich, C.W., Matyjaszczyk, M.S.: Mass spectrometric characterization of a high-field asymmetric waveform ion mobility spectrometer. Rev. Sci. Instrum. 69, 4094–4104 (1998)
Schneider, B.B., Covey, T.R., Coy, S.L., Krylov, E.V., Nazarov, E.G.: Chemical effects in the separation process of a differential mobility/mass spectrometer system. Anal. Chem. 82(5), 1867–1880 (2010)
Shvartsburg, A.A.: Differential Ion Mobility Spectrometry: Nonlinear Ion Transport and Fundamentals of FAIMS. CRC Press, Boca Raton (2008)
Vidal-de-Miguel, G., Macía, M., Cuevas, J.: Transversal modulation ion mobility spectrometry (TM-IMS), a new mobility filter overcoming turbulence-related limitations. Anal. Chem. 84, 7831–7837 (2012)
Vidal-de-Miguel, G., Macía, M., Barrios, C., Cuevas, J.: Transversal modulation ion mobility spectrometry (IMS) coupled with mass spectrometry (MS): exploring the IMS-IMS-MS possibilities of the instrument. Anal. Chem. 87(3), 1925–1932 (2015)
Gillig, K.J., Chen, C.H.: Increasing the performance of portable ion mobility analyzers: development of the periodic focusing differential mobility analyzer (PFDMA), Mass Spectrom (Mass Spectrometry Society of Japan, Tokyo, Japan), 3, Special Issue S0032 (2014). See also Gillig K.J. and Chen, C.H.: Periodic field differential mobility analyzer. US Patent Application Publication US 2013/0187042 A1, Jul. 25 (2013)
Kurulugama, R.T., Nachtigall, F.M., Lee, S., Valentine, S.J., Clemmer, D.E.: Overtone mobility spectrometry: part 1. Experimental observations. J. Am. Soc. Mass Spectrom. 20(5), 729–737 (2009)
Knutson, E., Whitby, K.: Aerosol classification by electric mobility: apparatus, theory, and applications. J. Aerosol Sci. 6, 443–451 (1975)
Labowsky, M., Fernandez de la Mora, J.: Novel ion mobility analyzers and filters. J. Aerosol Sci. 37(3), 340–362 (2006)
Rus, J., Moro, D., Sillero, J.A., Royuela, J., Casado, A., Fernández de la Mora, J.: IMS-MS studies based on coupling a differential mobility analyzer (DMA) to commercial API-MS systems. Int. J. Mass Spectrom. 298, 30–40 (2010)
Hogan, C.J., Ruotolo, B., Robinson, C., Fernandez de la Mora, J.: Tandem differential mobility analysis-mass spectrometry reveals partial gas-phase collapse of the GroEL complex. J. Phys. Chem. B 115(13), 3614–3621 (2011)
Larriba-Andaluz, C., Fernandez de la Mora, J.: Gas-phase structure of Coulombically stretched polyethylene glycol ions. J. Phys. Chem. B 116, 593–598 (2012)
Criado, E., Fernández-García, J., Fernández de la Mora, J.: Mass and charge distribution analysis of large polyethylene glycol chains by negative electrospray ion mobility mass spectrometry (NES-IMS-MS). Anal. Chem. 85(5), 2710–2716 (2013)
Fernández-García, J., Fernández de la Mora, J.: Measuring the effect of ion-induced drift-gas polarization on the electrical mobilities of multiply-charged ionic liquid nanodrops in air. J. Am. Soc. Mass Spectrom. 24, 1872–1889 (2013)
Javaheri, H., Le Blanc, Y., Thomson, B.A., Fernandez de la Mora, J., Rus, J., Sillero-Sepúlveda, JA.: Analytical characteristic of a differential mobility analyzer coupled to a triple quadrupole system (DMA-MSMS), poster 061, in: proceedings of the Annual ASMS Conference. Denver, 1–6 June (2008) Available at: http://www.seadm.com/descargas/ASMS_2008_DMA_Poster_Bruce_V3.pdf)
Zamora, D., Amo-Gonzalez, M., Lanza M., Fernández de la Mora, G., Fernández de la Mora, J.: Reaching a vapor sensitivity of 0.01 parts per quadrillion in the screening of large volume freight. [submied to Anal. Chem., March/3/2017] Also, Amo, M., Zamora, D., Casado, A., Fernandez de la Mora, G., Vidal-de-Miguel, G., Fernandez de la Mora, J.: Method for detecting atmospheric vapors at parts per quadrillion (ppq) concentrations, US Patent 9,297,785, 29 March (2016)
Vidal-de-Miguel, G., Macia, M., Pinacho, P., Blanco, J.: Low-sample flow secondary electrospray ionization: improving vapor ionization efficiency. Anal. Chem. 84(20), 8475–8479 (2012)
French, J.B., Reid, N.M., Buckley, J.A.: Method and apparatus for analyzing trace components using a gas curtain. US Patent 4,137,750 (1979)
Thomson, B.A., Iribarne, J.V.: Field-induced ion evaporation from liquid surfaces at atmospheric pressure. J. Chem. Phys. 71(11), 4451–4463 (1979)
Yamashita, M., Fenn, J.B.: Electrospray ion source, another variation of the free jet theme. J. Phys. Chem. 88(20), 4459–4465 (1984)
Amo, M., Fernández-de la Mora, J.: Limits to the chemical background and the mobility-selected current transmitted in a differential mobility analyser (DMA). Proceedings of the 62nd ASMS Conference on Mass Spectrometry and Allied Topics; Baltimore, Jun (2014)
Maißer, A., Thomas, J.M., Larriba-Andaluz, C., He, S., Hogan Jr., C.J.: The mass-mobility distributions of ions produced by a Po-210 source in air. J. Aerosol Sci. 90, 36–50 (2015)
Fernandez de la Mora, J.: Ionization of vapor molecules by an electrospray cloud. Int. J. Mass Spectrom. 300, 182–193 (2011)
Acknowledgements
The authors thank Dr. Anatoly Verenchikov for stressing the interest of improving the TR in DMA-MS coupling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Following Yale rules, Juan Fernandez de la Mora declares a financial interest in the company SEADM.
Rights and permissions
About this article
Cite this article
Amo-Gonzalez, M., Fernandez de la Mora, J. Mobility Peak Tailing Reduction in a Differential Mobility Analyzer (DMA) Coupled with a Mass Spectrometer and Several Ionization Sources. J. Am. Soc. Mass Spectrom. 28, 1506–1517 (2017). https://doi.org/10.1007/s13361-017-1630-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1630-2