Abstract
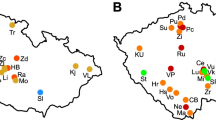
Powdery scab, caused by Spongospora subterranea, is an important potato disease. Greenhouse experiments in 2017/18 and 2018/19 on (very susceptible) ‘Agria’ seed tubers assessed if field-collected soils had different powdery scab-suppressive capabilities and identified factors involved in disease suppression. 2017/18: 12 geographically diverse soils with either S. subterranea added at planting or not added; 2018/19: six single-type soils used, to determine if powdery scab suppression was ‘general’, or ‘specific’ (transferable; possibly involving microorganisms), and if suppression was associated with soil physical, chemical, or biological factors (bacteria/fungi). For both seasons, S. subterranea soil ammendment increased scab severity on harvested tubers in all soils but one. Powdery scab severity (percent tubers with > 5% surface area covered by scabs) ranged from 0 to 39%. Soil texture, pH, soil organic matter and nutrient contents were associated with powdery scab incidence for some soils but not others. Effects of previous crop rotations on powdery scab were variable: one soil with three recent previous potato crops in rotation was disease-suppressive. All 2018/19 soils displayed some microbe-mediated disease suppression, three being more suppressive than others. Two had possible ‘specific’ Spongospora suppression (less disease when added to the conducive soil). Thus Spongospora-suppressive soils are present in New Zealand, and abiotic and biotic soil factors influenced incidence/severity of powdery scab of potato.


Similar content being viewed by others
References
Acosta-Martínez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770
Balendres MA, Tegg RS, Wilson CR (2017) Resting spore dormancy and infectivity characteristics of the potato powdery scab pathogen Spongospora subterranea. J Phytopathol 165:323–330
Ball BC, Bingham I, Rees RM, Watson CA, Litterick A (2005) The role of crop rotations in determining soil structure and crop growth conditions. Can J Soil Sci 85:557–577
Barillot CDC, Sarde CO, Bert V, Tarnaud E, Cochet N (2013) A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann Microbiol 63:471–476
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The second genome and plant health. Trends Plant Sci 17:478–486
Bollen GJ (1974) Fungal recolonization of heat-treated glasshouse soils. Agro-Ecosystems 1:139–155
Bonilla N, Gutiérrez-Barranquero J, Vicente A, Cazorla F (2012) Enhancing soil quality and plant health through suppressive organic amendments. Diversity 4:475–491
Brierley J, Lees A, Wale S (2008) Research review: Powdery scab – strains and conducive conditions. Potato Council, Oxford (UK). https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Potatoes/Powdery%20Scab%20Review%20Dec%202008.pdf. Accessed 26 July 2020
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Callahan B, McMurdie P, Rosen M, Han AW, Johnson AJ, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Campos SB, Lisboa BB, Camargo FAO et al (2016) Soil suppressiveness and its relations with the microbial community in a Brazilian subtropical agroecosystem under different management systems. Soil Biol Biochem 96:191–197
Cha JY, Han S, Hong HJ et al (2016) Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J 10:119–129
Cook RJ (2014) Plant health management: pathogen suppressive soils. Encyclopedia of agriculture and food systems. Elsevier, Amsterdam, pp 441–455
Cullen DW, Lees AK, Toth IK, Duncan JM (2002) Detection of Colletotrichum coccodes from soil and potato tubers by conventional and quantitative real-time PCR. Plant Pathol 51:281–292
Day PR (1965) Particle fractionation and particle size analysis. In: Black CA (ed) Methods of Soil Analysis, Part 1 Agronomy No. 9, American Society of Agronomy, Madison, Wisconsin, pp. 545-567
Egidi E, Delgado-Baquerizo M, Plett J et al (2019) A few Ascomycota taxa dominate soil fungal communities worldwide. Nat Commun 10:2369. https://doi.org/10.1038/s41467-019-10373-z
Falloon RE (2008) Control of powdery scab of potato: towards integrated disease management. Am J Potato Res 85:253–260
Falloon RE, Curtin D, Lister RA, Butler RC (2005) Different soil pHs have little effect on Spongospora subterranea infection of potato roots. Am J Potato Res 82:68–69
Falloon RE, Genet RA, Wallace AR, Butler RC (2003) Susceptibility of potato (Solanum tuberosum) cultivars to powdery scab (caused by Spongospora subterranea f. sp. subterranea) and relationships between tuber and root infection. Australas Plant Pathol 32:377–385
Falloon RE, Merz U, Butler RC, Curtin D, Lister RA, Thomas SM (2016) Root infection of potato (Solanum tuberosum) caused by Spongospora subterranea: knowledge review and evidence for decreased plant productivity. Plant Pathol 56:422–434
Falloon RE, Merz U, Lister RA, Wallace AR, Hayes SP (2011) Morphological enumeration of resting spores in sporosori of the plant pathogen Spongospora subterranea. Acta Protozool 50:121–132
Falloon RE, Viljanen-Rollinson SLH, Coles GD, Poff JD (1995) Disease severity keys for powdery and downy mildews of pea and powdery scab of potato. NZJ Crop Hortic Sci 23:31–37
Fiers M, Edel-Hermann V, Chatot C, Le Hingrat Y, Alabouvette C, Steinberg C (2012) Potato soil-borne diseases. A review. Agron Sustain Dev 32:93–132
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42:243–270
Gilchrist E, Soler J, Merz U, Reynaldi S (2011) Powdery scab effect on the potato Solanum tuberosum ssp. andigena growth and yield. Trop Plant Pathol 36:350–355
Girvan MS, Bullimore J, Pretty JN, Osborn AM, Ball AS (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol 69:1800–1809
Gomez Exposito R, de Bruijn I, Postma J, Raaijmakers JM (2017) Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front Microbiol 8:2529
Haas D, Defago G (2005) Biological control of soilborne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hoitink HAJ, Boehm MJ (1999) Biocontrol within the context of soil microbial communities: a substrate-dependent phenomenon. Annu Rev Phytopathol 37:427–446
Hutchison LJ, Kawchuk LM (1998) Spongospora subterranea f.sp. subterranea. Can J Plant Pathol 20:118–119
Igiehon NO, Babalola OO (2018) Rhizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. Int J Environ Res Public Health 15:576–610
Inceoglu O, Al-Soud WA, Falcao Salles J, Semenov AV, van Elsas JD (2011) Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS One. https://doi.org/10.1371/journal.pone.0023321
Jenkinson DS (1988) Determination of microbial biomass carbon and nitrogen in soil. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems CAB International, Wallingford, UK, pp 368–386
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucl Acids Res 41:e1–e1. https://doi.org/10.1093/nar/gks808
Koljalg U, Nilsson RH, Abarenkov K et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Larkin R, Griffin T (2007) Control of soilborne potato diseases using Brassica green manure. Crop Prot 26:1067–1077
Latz E, Eisenhauer N, Rall BC, Allan E, Roscher C, Scheu S, Jousset A (2012) Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities. J Ecol 100:597–604
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217–e61217
McMurdie PJ, Holmes S (2014) Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10:e1003531. https://doi.org/10.1371/journal.pcbi.1003531
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. https://doi.org/10.14806/ej.17.1.200
Martin KJ, Rygiewicz PT (2005) Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol 5:28
Mazzola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie Van Leeuwenhoek 81:557–564
Mazzola M (2007) Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39:213
Merz U (1989) Infectivity, inoculum density and germination of Spongospora subterranea resting spores: a solution-culture test system. Bull OEPP 19:585–592
Merz U (2008) Powdery scab of potato—occurrence, life cycle and epidemiology. Am J Potato Res 85:241–246
Merz U, Falloon RE (2009) Review: powdery scab of potato—increased knowledge of pathogen biology and disease epidemiology for effective disease management. Potato Res 52:17–37
Nacke H, Thurmer A, Wollherr A et al (2011) Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS One 6:e17000. https://doi.org/10.1371/journal.pone.0017000
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Bartels JM et al. (ed.) Methods of soil analysis: Part 3 Chemical methods, 3rd edn. ASA and SSSA Book Series 5, Madison, WI, USA, pp 961–1010
Nguyen NL, Kim YJ, Hoang VA, Subramaniyam S, Kang JP, Kang CH, Yang DC (2016) Bacterial diversity and community structure in Korean Ginseng field soil are shifted by cultivation time. PLoS One 11:e0155055. https://doi.org/10.1371/journal.pone.0155055
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, et al (eds) Methods of Soil Analysis, Part 2, 2nd edn, Agron Monogr 9. ASA and ASSA, Madison WI, pp 403–430
Ophel-Keller K, McKay A, Hartley D, Curran H, Curran J (2008) Development of a routine DNA-based testing service for soil borne diseases in Australia. Australas Plant Pathol 37:243–253
Orr CH, Stewart CJ, Leifert C, Cooper JM, Cummings SP (2015) Effect of crop management and sample year on abundance of soil bacterial communities in organic and conventional cropping systems. J Appl Microbiol 119:1364–5072
Penton CR, Gupta VVSR, Tiedje JM, Neate SM, Ophel-Keller K, Gillings M, Harvey P, Pham A, Roget DK (2014) Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS One 9:e93893
Peralta AL, Sun YM, McDaniel MD, Lennon JT (2018) Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 9:e02235
Postma J, Scheper RWA, Schilder MT (2010) Effect of successive cauliflower plantings and Rhizoctonia solani AG 2–1 inoculations on disease suppressiveness of a suppressive and a conducive soil. Soil Biol Biochem 42:804–812
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Raaijmakers JM, Mazzola M (2012) Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol 50:403–424
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Rovira AD, Wildermuth GB (1981) The nature and mechanism of suppression. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic Press, London, pp 385–415
Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T (2017) Disease suppressive soils: new insights from the soil microbiome. Phytopathol 107:1284–1297
Siegel-Hertz K, Edel-Hermann V, Chapelle E, Terrat S, Raaijmakers JM, Steinberg C (2018) Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Chateaurenard region. Front Microbiol 9:1–16
Simango K, van der Waals JE (2017) Effects of different soil treatments on the development of Spongospora subterranea f. sp. subterranea in potato roots and tubers in the greenhouse. Potato Res 60:47–60
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton PL (1990) Estimates of soil microbial C by a fumigation-extraction method: use on soils of high organic matter content, and a reassessment of the KEC factor. Soil Biol Biochem 22:301–307
Stagnitti F (2015) Parent Project for APRP2 program: final report hort innovation: PT09039. Horticulture Innovation Australia Limited. Sydney, Australia. https://ausveg.com.au/app/data/technical-insights/docs/PT09039.PDF. Accessed 19 September 2020.
Stirling GR, Smith MK, Smith JP, Stirling AM, Hamill SD (2012) Organic inputs, tillage and rotation practices influence soil health and suppressiveness to soilborne pests and pathogens of ginger. Australas Plant Pathol 41:99–112
Thangavel T, Tegg RS, Wilson CR (2015) Monitoring Spongospora subterranea development in potato roots reveals distinct infection patterns and enables efficient assessment of disease control methods. PLoS One 10:e0137647
Tuncer G (2002) The effect of irrigation and nitrogen on powdery scab and yield of potatoes. Potato Res 45:153–161
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Van de Graaf P, Lees AK, Cullen DW, Duncan JM (2003) Detection and quantification of Spongospora subterranea in soil, water and plant tissue samples using real-time PCR. Eur J Plant Pathol 109:589–597
Van de Graaf P, Lees AK, Wale SJ, Duncan JM (2005) Effect of soil inoculum level and environmental factors on potato powdery scab caused by Spongospora subterranea. Plant Pathol 54:22–28
Van de Graaf P, Wale SJ, Lees AK (2007) Factors affecting the incidence and severity of Spongospora subterranea infection and galling in potato roots. Plant Pathol 56:1005–1013
Van de Haar J (2000) The powdery scab situation in the Netherlands. In: Merz U, Lees AK (eds) Proceedings of the first European powdery scab workshop. Aberdeen, Scotland, pp 21–22
Van Os GJ, Van Ginkel JH (2001) Suppression of Pythium root rot in bulbous iris in relation to biomass and activity of the soil microflora. Soil Biol Biochem 33:1447–1454
Weller DM, Raaijmakers JM, McSpadden BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Wright PJ, Falloon RE, Hedderley D (2015) Different vegetable crop rotations affect soil microbial communities and soilborne diseases of potato and onion: literature review and a long-term field evaluation. NZJ Crop Hortic Sci 43:85–110
Acknowledgements
This research was funded by Horticulture Innovation Australia Limited (Hort Innovation), with co-investment from Potatoes New Zealand Incorporated. We thank vegetable growers’ who gave us permission to collect soils from their fields. Dr Kathy Ophel-Keller, Russell Burns and Danielle Giblot-Ducray (South Australian Research and Development Institute) provided soil tests for potato pathogen DNA, and Ngaire Foster (Manaaki Whenua – Landcare Research) carried out soil respiration tests, microbial biomass-C and -N measurements, and soil particle size determinations. Dr Farhat Shah prepared Spongospora subterranea inoculum for experiments; Shea Addison and Robyn White assisted with the DNA extractions; and Moe Jeram gave technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, P.J., Falloon, R.E., Anderson, C. et al. Factors influencing suppressiveness of soils to powdery scab of potato. Australasian Plant Pathol. 50, 715–728 (2021). https://doi.org/10.1007/s13313-021-00822-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-021-00822-z