Abstract
Mobility after spinal cord injury (SCI) is among the top goals of recovery and improvement in quality of life. Those with tetraplegia rank hand function as the most important area of recovery in their lives, and those with paraplegia, walking. Without hand function, emphasis in rehabilitation is placed on accessing one’s environment through technology. However, there is still much reliance on caretakers for many activities of daily living. For those with paraplegia, if incomplete, orthoses exist to augment walking function, but they require a significant amount of baseline strength and significant energy expenditure to use. Options for those with motor complete paraplegia have traditionally been limited to the wheelchair. While wheelchairs provide a modified level of independence, wheelchair users continue to face difficulties in access and mobility. In the past decade, research in SCI rehabilitation has expanded to include external motorized or robotic devices that initiate or augment movement. These robotic devices are used with 2 goals: to enhance recovery through repetitive, functional movement and increased neural plasticity and to act as a mobility aid beyond orthoses and wheelchairs. In addition, lower extremity exoskeletons have been shown to provide benefits to the secondary medical conditions after SCI such as pain, spasticity, decreased bone density, and neurogenic bowel. In this review, we discuss advances in robot-guided rehabilitation after SCI for the upper and lower extremities, as well as potential adjuncts to robotics.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) affects 17,700 people annually in the USA with a prevalence of about 300,000 people [1]. Worldwide incidence is estimated at between 250,000 and 500,000 annually [http://www.who.int/mediacentre/factsheets/fs384/en/]. The goal of this narrative review is to summarize the current robotic rehabilitation devices being utilized in the rehabilitation of individuals with SCI as well as the scientific literature investigating their efficacy. The majority of articles found were small sample size studies or case reports; therefore, rather than definitively outlining proven benefits, we discuss emerging trends that are beginning to be noted and evaluated. The field of robotics and spinal cord injury is relatively new, and therefore, to report here on these early findings can provide direction for future studies. Both upper and lower extremity devices are reviewed here, and some potential adjunct therapies to robotic neurorehabilitation are also considered.
Upper Extremity Robotics
Cervical SCI can result in partial or complete tetraplegia. The higher and more complete the injury to the cervical spinal cord, the more pronounced the paralysis of the arms, wrists, and fingers will be. In this population, arm and hand function consistently ranks highest in priority of recovery [2]. Each small improvement in motor control of the upper extremities can translate to significant ameliorations in function and increased independence for the individual. Subsequently, rehabilitation for the upper extremities typically focuses on strengthening any intact motor pathways to the arm and hand. There are a number of different robotic devices currently used for neurorehabilitation of the upper extremities following SCI. These devices typically target either the shoulder and elbow, or the wrist and fingers, and can be categorized as either exoskeletons or robotic end-effectors. Exoskeletons are devices that align with and support the articulation of targeted joint(s), whereas robotic end-effectors simply contact users at the distal part of their limb [3, 4]. Both of these robot types can be used to deliver a high volume of high quality movement repetitions, which promote functional recovery and may potentially facilitate adaptive plasticity [5, 6]. Both also have the capability to reduce therapist burden and cost of care [7]. There are currently 6 robotic devices on the market as upper limb rehabilitation tools: Armeo, InMotion ARM/WRIST, The Hand of Hope, ReoGo, and the Arm Light Exoskeleton (ALEx). See Table 1 for a description of the devices. We will describe these devices and discuss the literature surrounding their role in SCI rehabilitation.
Armeo devices (Hocoma AG, Volketswil, Switzerland) [https://www.hocoma.com/us/solutions/armeo-power/] were the first commercially available unilateral upper extremity exoskeletons for combined hand and arm rehabilitation. The devices include ArmeoPower for the most impaired users, ArmeoSpring, ArmeoSpring Pediatrics, and ArmeoSenso for the least impaired. The Armeo system is a well-documented and well-researched device and is the only device shown to lead to faster recovery after stroke compared to standard care [8]. In SCI, there are 2 studies which demonstrate the utility of this device for SCI rehabilitation. Rudhe et al. [9] demonstrated that the Armeo Spring is reliable as a clinical tool to measure movement workspace (subject seated in a chair, device is aligned on the arm, and area of movement is measured in the x (right-left), y (up-down), and z (far-close) axes) and to measure functional changes in the upper extremity during rehabilitation and can be clinically applicable within the Spinal Cord Independence Measure version III (SCIM III) (n = 28; ≥ 3 months; AIS C or D). Furthermore, Zariffa et al. demonstrated a relationship between clinical assessments of function and measurements from the Armeo Spring that further supports the use of these devices in a clinical setting without the need for a dedicated assessment tool [10]. It is important to note that these are still only pilot trials and that further studies are needed to demonstrate efficacy.
InMotion (Bionik Laboratories Corp., Toronto, Canada) [https://www.bioniklabs.com/products/inmotion-arm, https://wwwbioniklabs.com/products/inmotion-wrist ] is a commonly used fixed frame upper limb rehabilitation robot. It was designed to provide high intensity and reproducible upper limb rehabilitation in adults and older children. The device is able to deliver assistance to user-initiated movements or perform movements with no user input. The InMotion WRIST can be used independently as a stand-alone device or combined with the ARM feature. This device has a visual feedback component where interactive games can be used to increase the quality of therapy sessions as well as keep the user engaged, according to the manufacturer. Research using this device for SCI neurorehabilitation is limited; however, 1 study by Cortes et al. [11] (n = 10; > 1 year; AIS A to D) demonstrated that after a 6-week (1 h/day, 3 times/week) training regimen, significant improvements in both aim and smoothness of movement were found. There were, however, no significant changes in upper-extremity strength, pain, or spasticity.
The Hand of Hope (HoH) (Rehab-Robotics Company Ltd., Hong Kong, China) is a neuromuscular rehabilitation exoskeleton designed to help users regain hand mobility. The hand and forearm are strapped into this molded device, which uses electromyography sensors on the forearm to control the hand for a number of different tasks. The HoH also has a visual feedback component: using biofeedback techniques to report to users the strength of their activated target muscles. This system was designed mainly for the adult population; however, children can also use the device so long as their hands meet the minimum size requirements. The HoH engages users by assisting them through the following activities: hand closing and opening; thumb, index finger, and middle finger closing/opening; and middle, ring, and little finger closing and opening. This device has predominantly been used in the stroke population [12, 13]; however, 1 case report investigated the feasibility and effectiveness of training with this device in a 56-year-old individual who sustained a SCI (AIS C) 26 years prior to inclusion in the trial [14]. The participant engaged in 20 2-h rehabilitation visits over a 10-week period using the HoH and demonstrated some improvements in clinical outcomes following completion of the intervention. Although pinch force did not improve over the course of the trial, the participant demonstrated a 45% increase in grip strength, a 10% improvement in his Graded and Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) score as well as a 29% improvement in speed of completion, and a 22% improvement in the Box and Block test.
ReoGo™ (Motorika Medical, Caesarea, Israel) is a stationary fixed based, end-effector arm rehabilitation robot, which is capable of producing a wide range of reproducible movements in three-dimensional space. The ReoGo allows for movements at the elbow (flexion/extension), wrist (flexion/extension), and shoulder (flexion/extension; abduction/adduction; internal/external rotation). The ReoGo uses a real-time visual feedback monitor to display tasks and games for the user to perform. It has primarily been used for upper limb rehabilitation poststroke [15]; however, a review of the literature revealed a single case study in SCI. This case study followed a 51-year-old man with an acute incomplete SCI (26 days; AIS D) who incorporated the ReoGo into a traditional inpatient therapy regimen for a total of 20 days (1 h of ReoGo per day) [16]. This individual demonstrated noticeable improvements in muscle strength (shoulder internal and external rotators measured using manual muscle testing), active range of motion (shoulder external rotation and elbow flexion/extension), perceived right upper extremity function, and Functional Independence Measure (FIM™) self-care performance. However, since this is a single case study, and this was the acute rehabilitation period where it is difficult to distinguish the benefit from a rehabilitation intervention from the natural course of recovery, no specific conclusions on the efficacy or feasibility of this device for incorporation into standard of practice is possible at this stage.
ALEx (Kinetek Wearable Robotics, Ghezzano Pl, Italy) is a stationary, fixed frame exoskeleton for the whole arm that can reproduce the same movement path with integrated visual feedback (video games and virtual reality) [http://www.wearable-robotics.com/kinetek/products/alex/]. The ALEx provides guided assistance for complex upper limb movements, where the level of assistance can be titrated to the user’s needs. This device is capable of engaging both arms at the same time, thus allowing for bilateral rehabilitation training. To our knowledge, the ALEx has not yet been featured in any published studies of SCI neurorehabilitation.
Overall, these upper extremity devices have not been thoroughly studied in SCI populations and therefore lack strong scientific evidence to support their use. Furthermore, there appears to be little consensus on an appropriate outcome measure for upper extremity function for persons with tetraplegia. This gap in knowledge translates to an inadequate ability to capture small changes in physiological outcomes. More robust trials and established outcome measures can potentially be used, with further research and development, to develop therapies that promote large functional gains [17].
Lower Extremity Robotics
Thoracic and lower SCIs can result in partial to complete paralysis of the lower extremities, and recovery can be limited. Independent mobility for many can only be achieved at a wheelchair level, although walking oftentimes remains a priority for recovery and improved quality of life [18]. Powered exoskeleton devices have emerged as potential upright mobility devices for those with both incomplete or complete paralysis. We will refer to ambulation with the use of exoskeleton as exoskeleton-assisted walking (EAW). Other conventional rehabilitation methods such as bracing with orthoses involve high physical demand on users, therapists, and support staff needed to train and use orthoses [19]. Even body weight supported treadmill training (BWSTT) is limited as it is not portable and therefore not useful for community ambulation. The benefits of BWSTT, such as the Lokomat (Hocoma, Switzerland), as a rehabilitation tool have been outlined and challenged extensively [20], and will not be discussed in this section, but will be discussed as an adjunct robotic rehabilitative technique in the subsequent section. Powered exoskeletons have even been put forth as rehabilitation devices that can facilitate recovery by delivering fully weight-bearing, repetitive, symmetric locomotor training efficiently and in high volume [19]. Intense locomotor training may facilitate neuroplasticity, as has been shown in animal models [21,22,23,24] in a dose-dependent manner [24,25,26]. Given that the development and use of powered exoskeletons is relatively new, the current evidence supporting their use and purported benefits has mostly been limited to single-intervention trials with few participants or single case reports. Here, we discuss these beneficial trends, keeping in mind that sample sizes are small, controlled trials on a large scale have not been conducted, trends can be based on subjective survey data rather than objective measures, and that therefore definitive conclusions cannot be reached. We present in 1 place a collection of the purported benefits using rigid, powered exoskeletons of the lower extremity with scope narrowed to the systems available in the USA between 2010 and 2018 that facilitate ambulation in individuals with both complete and incomplete SCI without body weight support. The benefits addressed include strengthening impaired muscles, improving walking speed and efficiency, and addressing secondary medical conditions following SCI such as spasticity [27,28,29], pain [28, 30, 31], changes in the cardiovascular system and metabolism [32,33,34,35,36], bowel [27, 35, 37], bone [34], and overall quality of life [28, 30, 38].
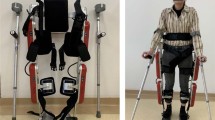
Currently, there are 3 such systems that are approved by the US Food and Drug Administration for use in the USA: The ReWalk™ (ReWalk Robotics, Inc., Marlborough, MA, USA) [http://rewalk.com/ ], Ekso® (Ekso Bionics, Richmond, CA, USA) [https://eksobionics.com/], and the Indego™ (Parker Hannifin Corp., Cleveland, OH, USA) [http://www.indego.com/indego/en/home]. Each system has specific inclusion and exclusion criteria and has been tested in different settings. All are class II medical devices. The systems by ReWalk and Indego have been approved for community and institutional use while Ekso has been approved for use within a medical institution with trained medical supervision only. Not approved in the USA but under development and in use in trials are 9 systems: Rex (Rex Bionics Ltd., Aukland, New Zealand) [https://www.rexbionics.com/], Exo-H2 (Technaid S.L., Madrid, Spain) [http://www.technaid.com/products/robotic-exoskeleton-exo-exoesqueleto/], Hank (Gogoa, Gipuzkoa, Spain) [http://gogoa.eu/], Arke™ (Bionik Laboratories Corp., Toronto, Canada) [https://www.bioniklabs.com/], Phoenix (SuitX, Berkely, CA, USA) [http://www.suitx.com/], ExoAtlet (ExoAtlet, Moscow, Russia) [https://www.exoatlet.com/en], Atlas 2020 and Atlas 2030 (Marsi-Bionics, Madrid, Spain) [http://www.marsibionics.com/], Axosuits® (Axosuits, Oradea, Romania) [http://www.axosuits.com/], and NASA-IHMC X1 Mina Exoskeleton (Institute for Human and Machine Cognition, Pensacola, FL, USA) [http://robots.ihmc.us/x1-mina-exoskeleton/]. See Table 2 for descriptions of the reviewed exoskeletons.
Several individual studies on specific exoskeletons and feasibility have been conducted. For example, the systems ReWalk [27, 29, 39,40,41,42], Ekso [39, 43, 44], Indego [45, 46], Exo-H2 [47], Arke [48], and X1 Mina [49] have been found to be practical for use. In addition to investigations of feasibility, several reviews have explored outcomes that relate to the efficacy of EAW. These reviews compare different exoskeleton systems as tools for rehabilitation in the chronic SCI population [50,51,52,53,54,55,56,57]. Parameters measured include time and frequency of sessions, steps taken, distance covered, time to don and doff devices, and overall competency with use. In addition, they evaluate multiple functional outcomes including the 6-min walk test, 10-m walk test, the Walking Index for Spinal Cord Injury version II (WISCI II) [58], the Spinal Cord Independence Measure (SCIM II) [59], Functional Independence Measure (FIM), and Timed Up and Go (TUG). Of these reviews, only Fisahn et al. [54] did not find EAW to be superior to conventional methods including BWSTT with or without electrical stimulation, stretching, and strength training; although the reviewed trials mostly looked at early generation exoskeletons prior to advanced modifications.
One meta-analysis by Miller et al. [60] investigated the safety and efficacy of the devices; including 14 studies (8 ReWalk™, 3 Ekso™, 2 Indego®, and 1 unspecified exoskeleton) with a total of 111 patients. The authors conclude that exoskeletons are safe to use and can contribute significant health benefits. However, the meta-analysis was later challenged with whether it contained duplicate subjects and studies, calling into question its results [61]. The following discussion evaluates many of the purported benefits and risks of EAW.
Spasticity
Spasticity is a common symptom after upper motor neuron spinal cord injury, defined as an increase in muscle tone and tendon reflexes [62]. Based on population sample studies, it has been found that from 66 to 78% of people [63, 64] reported spasticity following SCI, with a subset of, in 1 study, 40% reporting spasticity to be problematic (due to restricting activities of daily life and/or causing pain) [64] and in the other, 49% received treatment [63]. Improvement in spasticity with the use of EAW has been posited based on benefits described with BWSTT [65]; however, some studies have also measured this directly. In an investigation by Stampacchia et al. [28], 21 individuals with SCI (2–330 months; AIS A, B, or D) were assessed for spasticity before and after 1 40-min session of walking (7–25 min) in a powered exoskeleton (Ekso). Spasticity was measured using the Modified Ashworth Scale (MAS) [66] at the hip, knee, ankle flexors and extensors; the Penn Spasm Frequency Scale [67]; and perceived level of spasticity was assessed with a Numeric Rating Scale of 0–10. Comparing pre- and post-values, scores on all 3 scales were significantly reduced. In another study involving EAW using the ReWalk [27], 3 out of 11 subjects (≥ 1 year; AIS A or B) responded affirmatively to the questionnaire item: “Use of the device has lessened the spasticity in my lower limbs.” The MAS was also measured before and after each session (13–26 sessions total), and for each subject, the number of sessions where spasticity improved/worsened was reported. In 5 out of 12 subjects, spasticity improved in more sessions than it worsened, and in 2 subjects, spasticity worsened in a greater number of sessions than it improved; however, no statistical analysis was reported. Kressler et al. [31] reported no change in spasticity over the course of 18 EAW sessions in 3 subjects (≥ 1 year; AIS A), although the baseline scores of all 3 subjects was very mild (0–1 on the MAS). One subject did have clonus at the bilateral ankle that improved to mild clonus at the midpoint and end of testing. Finally, in a study by Zeilig et al. [29], in response to a questionnaire item; “Training with the device diminished spasticity in my legs,” the 6 patients (3–7 years; AIS A or B) expressed a mean score of 3.71 on the Likert scale, which represented a positive answer between 3) somewhat agree, and 4) agree. Overall, evidence has been largely based upon studies with small sample sizes and subjective reporting, but might indicate a positive effect of EAW on spasticity in some individuals with SCI. Further clinical trials are required before any firm conclusions can be made.
Pain
Chronic pain after SCI has been found to be prevalent in approximately 4 out of 5 individuals with spinal cord injury [68] and can be difficult to treat [69, 70]. Treatment can include both pharmacological and nonpharmacological interventions such as conventional physical therapy. Because chronic pain has been found to be refractory to treatment in 5–37% of patients [71], the effect of powered exoskeletons on pain is of particular interest. Kressler et al. [31] reported that in the 3 participants in their study, all experienced a reduction in their moderate to severe at-level SCI neuropathic pain. Zeilig et al. [29] reported anecdotally and not part of the outcome measures in the study that 1 participant with severe chronic neuropathic pain at baseline had consistent improvement after each training session. Another example of pain reduction can be found in the Stampacchia et al. study from 2016 described above [28], where among the 12 subjects who reported pain prior to EAW, a statistically significant reduction in pain scores was seen.
Conversely, Kolakowsky-Hayner et al. [43] reported on pain caused by use of the Ekso powered exoskeleton in 7 individuals with SCI (≤ 2 years; AIS A). Some participants reported mild upper extremity pain/soreness, which the authors attributed to performance of a new exercise modality that uses the upper extremities for support. Additionally, 2 participants reported the presence of lower back pain with use of the exoskeleton, although it should be noted that the trial was conducted with an earlier model of Ekso, and hip extension range of motion is now more limited to prevent such pain. Overall, evidence is limited to case studies or investigations with small sample sizes, but may indicate a potential positive effect of EAW on neuropathic pain or a potential pain generator in the upper extremities, both issues worthy of further exploration.
Cardiovascular System
It has been found that persons with SCI have an increased and accelerated risk of cardiovascular disease, and within this population, cardiovascular disease has become a leading cause of death [72,73,74]. A major risk factor in cardiovascular disease is obesity which has a high prevalence in the SCI population due to decreased capacity for physical activity and exercise, decrease in lean body mass, and increase in fat mass including intramuscular adipose tissue. Obesity is part of a metabolic syndrome including abnormal lipid profiles and impaired glucose tolerance. Exercise is recommended as the first line to target these cardiovascular risk factors. Patients with SCI are limited in types of cardiovascular exercise due to paralysis and the tremendous exertion needed to use traditional gait orthoses such as hip-knee-ankle-foot orthoses and reciprocating gait orthoses; however, powered exoskeletons may provide a viable alternative.
It has been shown that EAW increases heart rate and oxygen consumption in a manner that can serve as an effective means of cardiovascular exercise [32, 33]. In a study by Asselin et al. [32], oxygen uptake (VO2) and heart rate were recorded from 8 individuals with paraplegia (1.5–14 years; AIS A or B) during a 6-min walk test of EAW using the ReWalk. Average VO2 and heart rate were significantly higher during walking than either sitting or standing; reaching approximately half of their maximal heart rate reserve, which represents a moderate level of exercise intensity. The observed rise in VO2 also approached the American College of Sports Medicine’s (ACSM) recommendation for minimal training threshold [75]. Moreover, by recording VO2 from 5 subjects (mean, 11 years; AIS A) performing 6-min bouts of EAW using the Indego, Evans et al. [33] found EAW represented a moderate intensity exercise (3.0–5.9 metabolic equivalent of task units (METs)), which indicated that EAW may be beneficial to cardiovascular health. Similarly, Kozlowski et al. [35] showed that for 7 individuals with SCI (0.4–7.4 years; AIS A, B, or C) undertaking EAW with the Ekso device, elevations in heart rate, blood pressure and ratings of perceived exertion (RPE) [76, 77] were consistent with light to moderate exercise. However, Kressler et al. found that in 3 subjects (≥ 1 year; AIS A) who each completed 18 1-h sessions of EAW (3 sessions per week) energy expenditure during EAW indicated the activity was of light to very light exercise intensity and was not enough to be a sufficient source of exercise [31]. This study also showed that glucose and lipid markers did not change consistently during the course of the trial, but insulin resistance decreased consistently from baseline to the last week of training [31]. In a follow-up study by Kressler et al. [36] involving 6-min bouts of high-intensity EAW, 4 individuals with SCI (2–4 years; AIS not reported) demonstrated a wide variation of energy expenditure that differed with the device’s mode of assistance and participant characteristics. The device could be run in variable assist mode which allows the clinician to set the percent work the exoskeleton performs and allows for some participation from users based on strength and ability. Therefore, Kressler et al. [36] suggested that EAW could provide moderate intensity exercise when operating the Ekso powered exoskeleton in variable assist mode, which involves a higher work rate for the user, as well as for users who are more impaired and less fit. Of note, the Kozlowski and Kressler studies used a first-generation Ekso which provides upper body support through a rigid backpack and also required ceiling rail tether per manufacturer and used a front wheeled walker for support. The Evans study used the Indego which only rises up to the lower torso. In summary, EAW may provide a means of regular exercise beneficial to cardiovascular health, but this is based only on small sample sized cohort studies.
Change in Metabolism: Fat and Muscle
The possible benefits of a powered exoskeleton as an exercise tool have been noted above. If powered exoskeletons can provide a sufficient exercise stimulus, EAW might have the potential to improve metabolic health by decreasing total fat mass and increasing muscle mass. Karelis et al. [34] found 3 60-min sessions of EAW in the Ekso per week for 6 weeks led to an increase in leg and appendicular lean body mass, and a decrease in total, leg, and appendicular fat mass in 5 individuals with SCI (mean, 7.6 years; AIS A or B). However, total body weight and body mass index were increased. Future clinical trials of powered exoskeletons are needed that include outcome measures such as metabolic expenditure, obesity, insulin resistance, and lipid profiles so that these potentially beneficial effects of EAW can be further elucidated.
Bowel Function
Neurogenic bowel, including prolonged transit time and bowel dysmotility, is a common secondary medical complication after spinal cord injury [78]. The use of powered exoskeletons has been hypothesized to improve bowel function based on previous studies investigating the benefit of both upright posture and mobility on intestinal motility. One study involving 24 individuals with SCI (time since injury and AIS score not reported) compared the effects of EAW and BWSTT on bowel function, both of which involve an upright walking motion [37]. The results showed that 20 min of EAW (4 times per week for 1 month) on an unspecified exoskeleton significantly reduced both enema dose and defecation time (n = 12), whereas the same dose of BWSTT had no effect on bowel function (n = 12). Additionally, in a study conducted by Esquenazi et al. [27], subjects were asked to report on bowel function following 24 sessions in the ReWalk powered exoskeleton (60–90 min per session; approximately 3 sessions per week). In response to the questionnaire item; “During the period when I was using the device I felt an improvement in my intestinal activity,” 5 of 11 subjects (≥ 1 year; AIS A or B) reported improved bowel regulation. Similarly, in a study by Kozlowki et al. [35], 2 of 7 participants (0.4–7.4 years; AIS A, B, or C) noted more regular bowel movements that were easier to manage after 30 min of EAW using the Ekso. However, a study involving 6 individuals with SCI (> 6 months; AIS A or B) undergoing rehabilitation using the ReWalk found no improvement in reported bowel function in response to the questionnaire item: “I felt improvement in my bowel movement during the training program” [29]. Overall, some studies indicate a potential benefit of EAW on bowel function and some did not; however, future clinical trials of powered exoskeletons should include changes in gastrointestinal function in their outcome measures to further describe any potential benefits.
Quality of Life
Regaining function in the legs is 1 of the top priorities for patients with SCI [18]. EAW offers a realistic possibility of fulfilling this priority, even in patients with complete or incomplete paralysis. Therefore, the psychological effects of rehabilitation using powered exoskeletons have been investigated, with several studies demonstrating positive effects on quality of life scores. For example, 21 individuals with SCI (2–330 months; AIS A, B, or D) reported moderate positive changes in activity limitations, symptoms, emotions, and overall quality of life following 7–25 min of walking in the Ekso powered exoskeleton [28]. These participants also provided high scores on questionnaire items related to positive sensations/opinions and low scores for negative experiences, indicating a positive overall attitude towards powered exoskeletons. Moreover, in a 2-case report by Cruciger et al. [30], both subjects (10 and 19 years; AIS A) reported increased health-related quality of life scores for all 8 domains of the Short Form Health Survey (SF-36). Similarly, a case study involving 1 individual with paraplegia (1 year; AIS C) who was not independently ambulatory reported increased scores in 6 out of 8 areas of the SF-36 (physical functioning, physical role function, physical pain, general health, vitality, physical well-being) following 6 months of rehabilitation with the ReWalk powered exoskeleton [38]. Overall, the use of powered exoskeletons, both to stand upright and to walk, has consistently been shown to improve health-related quality of life measures in these few small studies.
Bone Health
Bone loss and the resulting osteoporosis are common secondary outcomes associated with SCI and occur at a rapid rate acutely after injury, before persisting at a slower rate for an extended period of time [79]. This bone loss predisposes individuals with SCI to fractures, especially at the distal femur and proximal tibia [79,80,81]. Bone loss is believed to be caused by a combination of factors including changes in bone metabolism due to hormonal deficiencies and blood-circulation abnormalities that can affect bone cell differentiation, as well as a reduction in mechanical forces from both weight-bearing activities and muscle contractions that promote the remodeling of bone [82]. Subsequently, it has been hypothesized that weight bearing in a device such as a powered exoskeleton may ameliorate the progressive loss of bone mineral density (BMD) [34]. To the authors’ knowledge there is only one published study documenting the effect of EAW on bone health following SCI [34]. In this study by Karelis et al. [34], 5 individuals with SCI (mean, 7.6 years; AIS A or B) who use a wheelchair as their primary mode of mobility were training on a powered exoskeleton (Ekso) for 6 weeks (3 sessions per week; up to 60 min per session). BMD total and of the leg (with no further specification regarding location) was measured with dual energy X-ray absorptiometry (DXA) and BMD of the right tibia at the 66% site (measured distal to proximal) was assessed with peripheral quantitative computed tomography (pQCT). These authors reported a nonsignificant 14.5% increase in BMD in the tibia as measured by pQCT. Measurements using DXA were much smaller in absolute value and also not statistically significant. There was 1 participant out of 5 who had an improvement in BMD which shifted his diagnosis from osteoporosis to osteopenia, although this participant’s individual raw data is not presented. It remains to be seen whether a greater training volume may elicit meaningful effects in preserving BMD following SCI.
Fractures
Risk of bone fractures has been associated with the use of powered exoskeletons due to decreased BMD coupled with unfamiliar forces placed on the paralyzed limbs during EAW, and/or the potential for misalignment and excessive torques of the exoskeleton during set-up and use coupled with a lack of pain response [83]. A previous meta-analysis by Miller et al. [60] found 9 out of the 14 reviewed studies reported on fractures, with 1 of the 9 reporting an occurrence of fracture. This equated to a 3.4% incidence of bone fracture with EAW. In order to decrease the risk of fracture, some SCI studies involving powered exoskeletons screen for osteoporosis, but the extent to which fracture is a risk at specific levels of BMD is unknown. There are efforts to determine fragility fracture risk stratification levels in the SCI population and comparing risk with already established tools used in the able-bodied population [84].
We found 2 published cases of fracture from use of the included reviewed powered exoskeleton in SCI individuals [41, 85], none of whom were screened for osteoporosis before study enrollment, although the risk of fracture is unknown with or without a diagnosis of osteoporosis. The first involved 10 participants (> 1 year; AIS A or C) who were to complete 20 sessions with the ReWalk in a 10-week period [41]. The subject who sustained the fracture (21.8 years; AIS A) presented with a swollen ankle the morning after the fourth training session and was found to have a hairline fracture of the talus. No treatment was required, but EAW was discontinued. Study eligibility criteria were subsequently adjusted to exclude those with a diagnosis of severe osteoporosis as identified by DXA or peripheral quantitative computed tomography (pQCT), although the location these measurements are taken and the numerical cut-offs are not defined. Severe osteoporosis according to the World Health Organization is defined as a DXA score of < 2.5 and the presence of at least 1 fragility fracture. In a recent study by Gagnon et al. [85], 1 subject (8.6 years; AIS A) out of 13 (0.8–31.4 years; AIS A or B) sustained bilateral calcaneal fractures after 2 familiarization sessions and 1 training session in the Ekso exoskeleton. Although the exact etiology is unknown, the authors pointed to increased fracture risk in the calcaneus due to elevated vertical ground reaction forces at heel strike during EAW [86]. This study by Gagnon et al. [85] did not have an exclusion criterion based upon BMD; participants would instead be excluded only if there was history of lower extremity fracture within the last year.
Both of these incidences of fractures [41, 85] involved individuals who sustained their SCI at least 5 years previously. The incidence rate of 3.4% provided by Miller et al. [60] may therefore underestimate the risk for individuals who are further postinjury, with more advanced levels of BMD loss.
Of the 14 studies in the meta-analysis by Miller et al. [60], only 3 started with objective measurable exclusion criteria for osteoporosis, and those criteria differed between studies: Esquenazi et al. [27] excluded osteoporosis as defined by a BMD t score via DXA below − 2.5 at the right-limb femoral neck and the L2–L4 spine; Yang et al. [87] excluded those with DXA results indicating a t score below − 3.0 at the lumbar spine and bilateral proximal femurs or knee BMD less than 0.70 g/cm2, and Spungen et al. [40] similarly excluded those with a knee (femoral neck or proximal tibia) BMD less than 0.70 g/cm2, or a hip t score less than − 3.0. Kozlowki et al. [35] reported a subjective, nonspecific exclusion criterion of “risk of bone fracture as determined by the physician screener.” As tallied in a separate review of 28 studies involving the Rex, HAL (a body weight-supported device), Ekso, ReWalk, and Indego [83], bone health was mentioned in 71% of the studies, but reporting was inconsistent. The threshold of exclusion differs between studies. It is yet unknown whether fracture risk can be decreased by initiating BMD screening, but efforts are underway to establish risk stratification guidelines [84]. Until guidelines are established, there is risk of excluding potential participants based on BMD criteria who are not at risk of fracture, although conversely with screening and BMD cut-offs, some fractures could potentially be avoided.
Ambulation
EAW can be an effective neurorehabilitation exercise, but is also touted as a potential alternative means of mobility for people with paralysis following SCI. In its current state, EAW still lags behind community ambulatory speeds. Reported walking speeds for the Ekso™ in a trial with 7 participants with 5 motor complete (AIS A or B), and with 1 participant C4 AIS B were between 0.11 and 0.21 m/s [35], with the C4 AIS B participant walking fastest and learning fastest, hypothesized to be due to his younger age and shorter duration of time since onset of injury. The studies involving the ReWalk™ included 1 with 6 participants with cervical to thoracic motor complete injuries that demonstrated significantly faster speeds on the 10-MWT the lower the injury [29], a 12 subject study with all complete thoracic level injuries that also demonstrated the same trend of performance influenced by injury level [27], and a study with 5 subjects, mixed incomplete and complete showed more improvement in walking speed in those with complete injury, with the final range in speeds from 0.25 to 0.48 m/s [27, 29, 41], In a trial with 16 participants, the Indego® demonstrated a range of 0.22 to 0.51 m/s with the fastest speeds corresponding to the lower levels of injury in a majority of motor complete (AIS A or B) participants [45]. All of these reported speeds in different devices, despite being target speeds that are difficult to reach, are below the commonly accepted threshold of 0.8 m/s for full community ambulation [50, 88]. Moreover, these speeds do not approach the required velocity to cross a road safely, approximately 1.06 m/s [89], which limits usage of these devices in the wider community. Also, not all individuals with spinal cord injury are able to navigate the technology and use of an exoskeleton device and therefore EAW would not be an option.
Robotics and Adjunct Methods
The field of rehabilitation robotics is growing rapidly and shows great promise due to the ability of robots to deliver both high-quality and quantity repetitions of therapeutically meaningful movements to patients. Although there is no substitute for high intensity physical therapy, there are certain adjuncts to rehabilitation that have been identified as rehabilitation “modulators”: techniques with the potential to enhance or multiply the effects of conventional rehabilitation [90]. Here, we will discuss the evidence surrounding some of these potential robotic neurorehabilitation modulators.
Noninvasive Brain Stimulation
Transcranial direct current stimulation (tDCS) causes subthreshold polarization of neuron membranes [91, 92] and is capable of modulating corticospinal excitability to muscles affected by SCI [93]. Moreover, tDCS may facilitate plasticity via polarity-specific modulation of both GABAergic and glutamatergic synaptic strength [94]. Thus, tDCS has the potential to modulate responses to robotic neurorehabilitation. tDCS has also shown the potential to improve motor function in chronic SCI as a single treatment methodology [95] and is therefore of particular interest to researchers and clinicians already using rehabilitation robotics.
Two recent studies have indicated that tDCS may have the potential to augment the therapeutic benefits of robot-assisted neurorehabilitation in chronic SCI individuals; 1 with tDCS applied as an adjunct to upper limb movement training [96] and 1 with tDCS applied in combination with EAW [96, 97]. Both studies involved a double-blind, randomized, sham-controlled trial design with subjects undertaking robot-assisted movement training immediately following 20 min of either active or sham tDCS. Yozbatiran et al. [96] found individuals with cervical SCI (> 6 months; AIS C or D) demonstrated greater improvement in arm and hand function scores on the Jebsen-Taylor Hand Function Test with active (n = 4) versus sham tDCS (n = 4) following 10 sessions of tDCS paired with upper extremity movement training using the MAHIExo-II exoskeleton. Similarly, Raithatha and colleagues [97] found traumatic SCI individuals (> 1 year; AIS B, C, or D) that received active tDCS (n = 9) demonstrated greater improvements in manual muscle testing scores for the right leg when compared to subjects in the sham tDCS group (n = 6) following 36 sessions of tDCS paired with locomotor training using the Lokomat. In contrast, 1 investigation found no additional benefit of tDCS as an adjunct to locomotor training in the Lokomat in 24 individuals with cervical or thoracic SCI (>1 year; AIS C or D) [98]. Each subject completed 20 sessions of locomotor training immediately after 20 min of either active or sham tDCS, over 4 weeks, during which time they continued to receive a comprehensive standard of care including 5 h of various rehabilitation therapies and fitness training per weekday. Both active and sham tDCS groups (n = 12) improved function after the 4-week trial; however, tDCS had no effect on lower extremity motor score, 10-m walk test or the WISCI II [98]. The cause for the discrepancy in the results of these 3 studies is unclear. One could speculate that the high volume of rehabilitation being undertaken by the subjects in the study by Kumru et al. [98] could have washed out any supplementary effects of tDCS on a small portion of this total rehabilitation. However, active tDCS had no effect on walking metrics, such as the 10-m walk test, in either study involving locomotor training [97, 98], and limb strength was not improved by active tDCS in 2 of the 3 studies [96, 98]. This variability in results is also reflective of the wider tDCS literature as a whole [99], including similar studies involving robotic neurorehabilitation paired with tDCS in stroke survivors [100]. The efficacy of tDCS remains to be established as a single treatment modality in SCI and more basic research on its effects on the nervous system are required before pairing with robotic neurorehabilitation is justified.
Repetitive transcranial magnetic stimulation (rTMS) is another noninvasive neuromodulation technique, which directly perturbs neuronal firing and ongoing network activity by evoking action potentials in the cortex underlying the TMS coil. rTMS can induce synaptic plasticity [101] and subsequently has the potential to modulate responses to robotic neurorehabilitation. Despite this, the use of rTMS as an adjunct to robotic rehabilitation has received little attention within the SCI literature.
Calabrò et al. [102] provide a case report of 1 subject with SCI who demonstrated substantial benefits of robot-assisted locomotor training paired with rTMS, compared to the robotic rehabilitation alone. In this report, a 31-year-old male who sustained an incomplete traumatic SCI 20 months previously (AIS C at enrollment) completed 40 40-min locomotor training sessions in the Lokomat over 8 weeks. After a 1-month interval, this entire protocol was repeated with the training performed immediately following rTMS on 9 visits over the first 3 weeks of the 8-week protocol. The robot-assisted training alone provided only a mild reduction of hip and knee stiffness, which returned to baseline levels after 1-month follow-up (prior to the second installment of training). Conversely, when paired with rTMS, the same training protocol resulted in a significant reduction of hip and knee stiffness, increased hip extension strength, and higher motor evoked potential amplitude. The subject’s lower extremity motor score was also increased from 3 to 9 (of a maximum 25; as the left and right legs were not scored separately), and he was reclassified as AIS D. Although impossible to rule out both time and order effects, and the lack of blinding contributing to the benefits observed following the robot-assisted locomotor training paired with rTMS, this report provides an intriguing indication of the potential benefits of rTMS as an adjunct therapy to robotic neurorehabilitation. Appropriately blinded randomized control trials are required to explore the effects of rTMS paired with robot-assisted rehabilitation on the restoration of motor function following SCI. This call for further investigation echoes those made by reviews of both rTMS alone and rTMS paired with robotic neurorehabilitation in stroke patients [103, 104].
Overall, tDCS and rTMS may have the potential to facilitate neuroplastic changes at the cortical and/or spinal levels when combined with existing robotic neurorehabilitation therapies, but insufficient evidence is currently available to advocate their use in clinical settings. Further research is needed with regard to dosing, application timing, cortical targeting, and underlying mechanisms of both tDCS and rTMS, which may enable interventions to be tailored to individual patients.
Brain Machine Interfaces
Brain machine interfaces (BMIs) involve the pairing of assistive devices with a brain signal recording device. This recording device may be surgically implanted within the individual’s skull or placed noninvasively over the scalp. The recorded brain signals are decoded and turned into an instruction to control the assistive device. BMI-controlled neuroprosthetics can replace lost motor function in humans [105, 106]. Ostensibly, BMIs can also be paired with robotic neurorehabilitation devices to restore function following SCI; however, this possibility has received a paucity of attention.
Donati et al. [107] conducted a 12-month multimodality rehabilitation program with 8 subjects with chronic complete paraplegia (3–13 years; AIS A or B); central to which was the involvement of electroencephalography-based BMI training to control a robotic gait orthosis (Lokomat), lower limb robotic exoskeleton, and a virtual reality avatar body. The goal of the program was to restore the ability to walk autonomously using a brain-controlled exoskeleton; however, all 8 of the subjects demonstrated clinically significant improvements in somatic sensation and voluntary motor control of muscles below their SCI, with half of the subjects subsequently upgraded to AIS C (incomplete SCI) at 12 months. The authors concluded that long-term practice of BMI-integrated neurorehabilitation may promote both cortical and spinal cord plasticity, capable of underpinning a partial neurological recovery; however, it is not clear how or to what extent each individual rehabilitation modality influenced these outcomes.
Combining BMIs with robotic orthoses might pair volitional efferent signals from the brain with physiologically relevant afferent signals generated by movements of the limbs in the robotic device (either actively or passively, depending on the extent of the SCI). This could facilitate the restoration of corticospinal transmission, as indicated by studies involving paired associative stimulation that have demonstrated plastic effects in both the cortex [108] and spinal cord [109], which are sufficient to improve motor function after SCI [110, 111]. Theoretically then, BMIs might provide an effective adjunct to robotic neurorehabilitation; however, for now, the prospect of BMI-controlled robotic neurorehabilitation devices for SCI remains a concept discussed more frequently in review articles (such as this one) than in original research papers investigating efficacy. Barriers to the adoption of these technologies include high cost and additional time requirements; however, the biggest limiting factor in the spreading of this technology may be the number of experts able to construct and implement these systems. Future research and development must progress beyond single subject, proof of concept studies, to include randomized control trials that can be overseen by trained, but not BMI expert, operators.
Gamification and Virtual Reality
In neurological rehabilitation, level of engagement during rehabilitation is known to have a significant effect on active participation [112], which is thought to promote cortical plasticity [113] and improve outcomes in neurological patients [114]. As such, integrating gamification techniques into existing models of neurorehabilitation to increase participant engagement has become a priority in the last few years [115]. Although the available literature is sparse, there is evidence to suggest that gamifying robotic rehabilitation can be beneficial in improving participant engagement [116,117,118]. In particular, the application of virtual reality technologies is emerging as an efficacious method of increasing active participation through gamification. Novel virtual reality environments have been demonstrated to enhance motor output in single sessions of robot-assisted gait rehabilitation in children with various neurological disorders [119,120,121]. Moreover, Zimmerli et al. [122] investigated the effects of virtual reality mediated gamification of lower limb robotic rehabilitation in adults with SCI. In a small correlational trial consisting of 22 participants (10 healthy controls and 12 with SCI 0.3–18.7 years; AIS A–D), Zimmerli and colleagues [122] found that more engaging virtual environments involving feedback on the walking speed of the controlled avatar and competition with virtual opponents resulted in significantly higher heart rate and muscle activity in the gastrocnemius medialis, rectus femoris, tibialis anterior, and biceps femoris muscles during training on the Lokomat robot. In stroke, 1 longitudinal randomized control trial compared 12 sessions of robot-assisted gait training either with (n = 11) or without (n = 14) the presentation of a virtual reality environment [123]. Although only a pilot trial with a small sample size, the results show a tendency for greater functional improvements in the virtual reality group, with consistent increases in lower limb muscle strength found only in the virtual reality intervention group [123]. Overall, gamification of robot-assisted rehabilitation using engaging and interactive virtual reality set-ups shows promise across multiple fields of neurorehabilitation and warrants further investigation in SCI populations.
Conclusions
There are many promising interventions using robotics to improve the mobility, function, and quality of life of those living with spinal cord injury. Continued research into the development of even more streamlined and seamlessly integrated technology is needed. Larger, more extensive studies of upper extremity robotics are needed to further explore their efficacy given the positive outcomes of the limited current literature. Studies of lower extremity robotic exoskeletons have been more extensive and have shown them to be feasible, safe, and deliver results in gait that could in the future begin to near comfortable walking speeds. With the development of new exoskeleton systems around the world, the potential for growth in this sector and further benefit on rehabilitation and mobility is just beginning. In addition, evidence points to the positive effect of lower extremity exoskeletons on secondary medical conditions following SCI, but further clinical study is required to delineate dose requirements and more objective outcomes. Already, robotic technology and the adjunct methods discussed above are being used therapeutically with positive outcomes, and that opens the door to push further and to develop more innovate ways to promote plasticity and recovery. The current state of neurorehabilitation with robotics is promising and the future exciting. With time, robotic technology for both upper and lower extremities could push the limits of SCI rehabilitation.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. In: Birmingham UoAa, editor. Birmingham, AL2018.
Lo C, Tran Y, Anderson K, Craig A, Middleton J. Functional Priorities in Persons with Spinal Cord Injury: Using Discrete Choice Experiments To Determine Preferences. Journal of neurotrauma. 2016;33(21):1958-68
Pehlivan AU, Sergi F, Erwin A, Yozbatiran N, Francisco GE, O'Malley MK. Design and validation of the RiceWrist-S exoskeleton for robotic rehabilitation after incomplete spinal cord injury. Robotica. 2014;32(8):1415-31.
Vanmulken D, Spooren A, Bongers H, Seelen H. Robot-assisted task-oriented upper extremity skill training in cervical spinal cord injury: a feasibility study. Spinal Cord. 2015;53(7):547.
Kadivar Z, Sullivan J, Eng D, Pehlivan A, O'malley M, Yozbatiran N, et al., editors. Robotic training and kinematic analysis of arm and hand after incomplete spinal cord injury: a case study. Rehabilitation Robotics (ICORR), 2011 IEEE International Conference on; 2011: IEEE.
Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain research bulletin. 2009;78(1):4-12.
Riener R. Rehabilitation robotics. Foundations and Trends® in Robotics. 2013;3(1–2):1-137.
Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. The Lancet Neurology. 2014;13(2):159-66.
Rudhe C, Albisser U, Starkey ML, Curt A, Bolliger M. Reliability of movement workspace measurements in a passive arm orthosis used in spinal cord injury rehabilitation. Journal of neuroengineering and rehabilitation. 2012;9:37.
Zariffa J, Kapadia N, Kramer JL, Taylor P, Alizadeh-Meghrazi M, Zivanovic V, et al. Relationship between clinical assessments of function and measurements from an upper-limb robotic rehabilitation device in cervical spinal cord injury. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2012;20(3):341-50.
Cortes M, Elder J, Rykman A, Murray L, Avedissian M, Stampas A, et al. Improved motor performance in chronic spinal cord injury following upper-limb robotic training. NeuroRehabilitation. 2013;33(1):57-65.
Hu XL, Tong KY, Wei XJ, Rong W, Susanto EA, Ho SK. The effects of post-stroke upper-limb training with an electromyography (EMG)-driven hand robot. J Electromyogr Kinesiol. 2013;23(5):1065-74.
Susanto EA, Tong RK, Ockenfeld C, Ho NS. Efficacy of robot-assisted fingers training in chronic stroke survivors: a pilot randomized-controlled trial. Journal of neuroengineering and rehabilitation. 2015;12:42.
Lu Z, Tong KY, Shin H, Stampas A, Zhou P. Robotic Hand-Assisted Training for Spinal Cord Injury Driven by Myoelectric Pattern Recognition: A Case Report. Am J Phys Med Rehabil. 2017;96(10 Suppl 1):S146-s9.
Takahashi K, Domen K, Sakamoto T, Toshima M, Otaka Y, Seto M, et al. Efficacy of Upper Extremity Robotic Therapy in Subacute Poststroke Hemiplegia: An Exploratory Randomized Trial. Stroke. 2016;47(5):1385-8.
Siedziewski L, Schaaf RC, Mount J. Use of robotics in spinal cord injury: a case report. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2012;66(1):51-8.
Mulcahey MJ, Hutchinson D, Kozin S. Assessment of upper limb in tetraplegia: considerations in evaluation and outcomes research. J Rehabil Res Dev. 2007;44(1):91-102.
Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(7):500-6.
Arazpour M, Bani MA, Hutchins SW, Jones RK. The physiological cost index of walking with mechanical and powered gait orthosis in patients with spinal cord injury. Spinal Cord. 2013;51(5):356-9.
Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94(11):2297-308.
See PA, de Leon RD. Robotic loading during treadmill training enhances locomotor recovery in rats spinally transected as neonates. J Neurophysiol. 2013;110(3):760-7.
de Leon RD, See PA, Chow CH. Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. Journal of neurotrauma. 2011;28(6):1021-33.
Macias M, Nowicka D, Czupryn A, Sulejczak D, Skup M, Skangiel-Kramska J, et al. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 2009;10:144.
Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, et al. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci. 2007;27(16):4460-71.
Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28(29):7370-5.
Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22(8):3130-43.
Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911-21.
Stampacchia G, Rustici A, Bigazzi S, Gerini A, Tombini T, Mazzoleni S. Walking with a powered robotic exoskeleton: Subjective experience, spasticity and pain in spinal cord injured persons. NeuroRehabilitation. 2016;39(2):277-83.
Zeilig G, Weingarden H, Zwecker M, Dudkiewicz I, Bloch A, Esquenazi A. Safety and tolerance of the ReWalk exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. The journal of spinal cord medicine. 2012;35(2):96-101.
Cruciger O, Schildhauer TA, Meindl RC, Tegenthoff M, Schwenkreis P, Citak M, et al. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: a case study (.). Disability and rehabilitation Assistive technology. 2016;11(6):529-34.
Kressler J, Thomas CK, Field-Fote EC, Sanchez J, Widerstrom-Noga E, Cilien DC, et al. Understanding therapeutic benefits of overground bionic ambulation: exploratory case series in persons with chronic, complete spinal cord injury. Arch Phys Med Rehabil. 2014;95(10):1878-87.e4.
Asselin P, Knezevic S, Kornfeld S, Cirnigliaro C, Agranova-Breyter I, Bauman WA, et al. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52(2):147-58.
Evans N, Hartigan C, Kandilakis C, Pharo E, Clesson I. Acute Cardiorespiratory and Metabolic Responses During Exoskeleton-Assisted Walking Overground Among Persons with Chronic Spinal Cord Injury. Topics in spinal cord injury rehabilitation. 2015;21(2):122-32.
Karelis AD, Carvalho LP, Castillo MJ, Gagnon DH, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49(1):84-7.
Kozlowski AJ, Bryce TN, Dijkers MP. Time and Effort Required by Persons with Spinal Cord Injury to Learn to Use a Powered Exoskeleton for Assisted Walking. Topics in spinal cord injury rehabilitation. 2015;21(2):110-21.
Kressler J, Wymer T, Domingo A. Respiratory, cardiovascular and metabolic responses during different modes of overground bionic ambulation in persons with motor-incomplete spinal cord injury: A case series. J Rehabil Med. 2018;50(2):173-80.
Huang Q, Yu L, Gu R, Zhou Y, Hu C. Effects of robot training on bowel function in patients with spinal cord injury. J Phys Ther Sci. 2015;27(5):1377-8.
Raab K, Krakow K, Tripp F, Jung M. Effects of training with the ReWalk exoskeleton on quality of life in incomplete spinal cord injury: a single case study. Spinal Cord Ser Cases. 2016;2:15025.
Bryce TN, Dijkers MP, Kozlowski AJ. Framework for Assessment of the Usability of Lower-Extremity Robotic Exoskeletal Orthoses. Am J Phys Med Rehabil. 2015;94(11):1000-14.
Spungen AM AP, Fineberg DB, Kornfeld SD, Harel NY. Exoskeletal-assisted walking for persons with motor-complete paraplegia. NATO Science and Technology Organization; Milan, Italy2013.
Benson I, Hart K, Tussler D, van Middendorp JJ. Lower-limb exoskeletons for individuals with chronic spinal cord injury: findings from a feasibility study. Clin Rehabil. 2016;30(1):73-84.
Asselin PK, Avedissian M, Knezevic S, Kornfeld S, Spungen AM. Training Persons with Spinal Cord Injury to Ambulate Using a Powered Exoskeleton. Journal of visualized experiments : JoVE. 2016(112).
Kolakowsky-Hayner SA CJ, Moran S, Shah A. Safety and feasibility of the EksoTM bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013;S4.
Bach Baunsgaard C, Vig Nissen U, Katrin Brust A, Frotzler A, Ribeill C, Kalke YB, et al. Gait training after spinal cord injury: safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018;56(2):106-16.
Hartigan C, Kandilakis C, Dalley S, Clausen M, Wilson E, Morrison S, et al. Mobility Outcomes Following Five Training Sessions with a Powered Exoskeleton. Topics in spinal cord injury rehabilitation. 2015;21(2):93-9.
Tefertiller C, Hays K, Jones J, Jayaraman A, Hartigan C, Bushnik T, et al. Initial Outcomes from a Multicenter Study Utilizing the Indego Powered Exoskeleton in Spinal Cord Injury. Topics in spinal cord injury rehabilitation. 2018;24(1):78-85.
Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. Journal of neuroengineering and rehabilitation. 2015;12:54.
Lemaire ED, Smith AJ, Herbert-Copley A, Sreenivasan V. Lower extremity robotic exoskeleton training: Case studies for complete spinal cord injury walking. NeuroRehabilitation. 2017;41(1):97-103.
Neuhaus PD, Noorden JH, Craig TJ, Torres T, Kirschbaum J, Pratt JE. Design and evaluation of Mina: a robotic orthosis for paraplegics. IEEE Int Conf Rehabil Robot. 2011;2011:5975468.
Chang SR, Kobetic R, Audu ML, Quinn RD, Triolo RJ. Powered Lower-Limb Exoskeletons to Restore Gait for Individuals with Paraplegia - a Review. Case Orthop J. 2015;12(1):75-80.
Federici S, Meloni F, Bracalenti M, De Filippis ML. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: A systematic review. NeuroRehabilitation. 2015;37(3):321-40.
Lajeunesse V, Vincent C, Routhier F, Careau E, Michaud F. Exoskeletons' design and usefulness evidence according to a systematic review of lower limb exoskeletons used for functional mobility by people with spinal cord injury. Disability and rehabilitation Assistive technology. 2016;11(7):535-47.
Louie DR, Eng JJ, Lam T. Gait speed using powered robotic exoskeletons after spinal cord injury: a systematic review and correlational study. Journal of neuroengineering and rehabilitation. 2015;12:82.
Fisahn C, Aach M, Jansen O, Moisi M, Mayadev A, Pagarigan KT, et al. The Effectiveness and Safety of Exoskeletons as Assistive and Rehabilitation Devices in the Treatment of Neurologic Gait Disorders in Patients with Spinal Cord Injury: A Systematic Review. Global spine journal. 2016;6(8):822-41.
Holanda LJ, Silva PMM, Amorim TC, Lacerda MO, Simao CR, Morya E. Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: a systematic review. Journal of neuroengineering and rehabilitation. 2017;14(1):126.
Esquenazi A, Talaty M, Jayaraman A. Powered Exoskeletons for Walking Assistance in Persons with Central Nervous System Injuries: A Narrative Review. PM R. 2017;9(1):46-62.
Contreras-Vidal JL, N AB, Brantley J, Cruz-Garza JG, He Y, Manley Q, et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J Neural Eng. 2016;13(3):031001
Dittuno PL, Ditunno JF, Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39(12):654-6.
Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J, et al. The Catz-Itzkovich SCIM: a revised version of the Spinal Cord Independence Measure. Disabil Rehabil. 2001;23(6):263-8.
Miller LE, Zimmermann AK, Herbert WG. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Medical devices (Auckland, NZ). 2016;9:455-66.
Dijkers MP, Akers KG, Galen SS, Patzer DE, Vu PT. Letter to the editor regarding "Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis". Medical devices (Auckland, NZ). 2016;9:419-21.
Lance J. “Symposium,” in Spasticity: Disordered Motor Control, Felman RG, Young RR, Koella WP (eds). Year Book Medical Publishers. 1980:485–95.
Maynard FM, Karunas RS, Waring WP, 3rd. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil. 1990;71(8):566-9.
Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80(12):1548-57.
Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. The journal of spinal cord medicine. 2011;34(5):488-94.
Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206-7.
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320(23):1517-21.
Cardenas DD, Bryce TN, Shem K, Richards JS, Elhefni H. Gender and minority differences in the pain experience of people with spinal cord injury. Arch Phys Med Rehabil. 2004;85(11):1774-81.
Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev. 2009;46(1):13-29.
Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: A survey study. The journal of spinal cord medicine. 2006;29(2):109-17.
Ravenscroft A, Ahmed YS, Burnside IG. Chronic pain after spinal cord injury: a survey of practice in UK spinal injury units. Spinal Cord. 1999;37(1):25-8.
Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466-76.
Hagen EM, Lie SA, Rekand T, Gilhus NE, Gronning M. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2010;81(4):368-73.
Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142-52.
American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-91.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-81.
Al-Rahamneh HQ, Eston RG. Prediction of peak oxygen consumption from the ratings of perceived exertion during a graded exercise test and ramp exercise test in able-bodied participants and paraplegic persons. Arch Phys Med Rehabil. 2011;92(2):277-83.
Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39(4):193-203.
Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11(1):109-40.
Ashe MC, Craven C, Eng JJ, Krassioukov A, the SRT. Prevention and Treatment of Bone Loss after a Spinal Cord Injury: A Systematic Review. Topics in spinal cord injury rehabilitation. 2007;13(1):123-45.
Smith E, Carroll A. Bone mineral density in adults disabled through acquired neurological conditions: a review. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2011;14(2):85-94.
Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord. 2006;44(4):203-10.
He Y, Eguren D, Luu TP, Contreras-Vidal JL. Risk management and regulations for lower limb medical exoskeletons: a review. Medical devices (Auckland, NZ). 2017;10:89-107.
Cervinka T, Lynch CL, Giangregorio L, Adachi JD, Papaioannou A, Thabane L, et al. Agreement between fragility fracture risk assessment algorithms as applied to adults with chronic spinal cord injury. Spinal Cord. 2017;55(11):985-93.
Gagnon DH, Escalona MJ, Vermette M, Carvalho LP, Karelis AD, Duclos C, et al. Locomotor training using an overground robotic exoskeleton in long-term manual wheelchair users with a chronic spinal cord injury living in the community: Lessons learned from a feasibility study in terms of recruitment, attendance, learnability, performance and safety. Journal of neuroengineering and rehabilitation. 2018;15(1):12.
Fineberg DB, Asselin P, Harel NY, Agranova-Breyter I, Kornfeld SD, Bauman WA, et al. Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. The journal of spinal cord medicine. 2013;36(4):313-21.
Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen AM. Assessment of In-Hospital Walking Velocity and Level of Assistance in a Powered Exoskeleton in Persons with Spinal Cord Injury. Topics in spinal cord injury rehabilitation. 2015;21(2):100-9.
Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982-9.
Lapointe R, Lajoie Y, Serresse O, Barbeau H. Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 2001;39(6):327-35.
Krakauer JW, & Carmichael, S. T. . Broken Movement: The Neurobiology of Motor Recovery After Stroke: MIT Press; 2017.
Purpura DP, McMurtry JG. Intracellular Activities and Evoked Potential Changes during Polarization of Motor Cortex. J Neurophysiol. 1965;28:166-85.
Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557(Pt 1):175-90.
Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A, et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil. 2015;96(4 Suppl):S114-21.
Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37-53.
Cortes M, Medeiros AH, Gandhi A, Lee P, Krebs HI, Thickbroom G, et al. Improved grasp function with transcranial direct current stimulation in chronic spinal cord injury. NeuroRehabilitation. 2017;41(1):51-9.
Yozbatiran N, Keser Z, Davis M, Stampas A, O'Malley MK, Cooper-Hay C, et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: A proof of concept sham-randomized clinical study. NeuroRehabilitation. 2016;39(3):401-11.
Raithatha R, Carrico C, Powell ES, Westgate PM, Chelette Ii KC, Lee K, et al. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study. NeuroRehabilitation. 2016;38(1):15-25.
Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat((R)) gait training. Neuroscience letters. 2016;620:143-7.
Heroux ME, Loo CK, Taylor JL, Gandevia SC. Questionable science and reproducibility in electrical brain stimulation research. PLoS One. 2017;12(4):e0175635.
Simonetti D, Zollo L, Milighetti S, Miccinilli S, Bravi M, Ranieri F, et al. Literature Review on the Effects of tDCS Coupled with Robotic Therapy in Post Stroke Upper Limb Rehabilitation. Frontiers in human neuroscience. 2017;11:268.
Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15(4):253-66.
Calabro RS, Naro A, Leo A, Bramanti P. Usefulness of robotic gait training plus neuromodulation in chronic spinal cord injury: a case report. The journal of spinal cord medicine. 2017;40(1):118-21.
Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014(11):CD010820.
Mazzoleni S, Duret C, Grosmaire AG, Battini E. Combining Upper Limb Robotic Rehabilitation with Other Therapeutic Approaches after Stroke: Current Status, Rationale, and Challenges. Biomed Res Int. 2017;2017:8905637.
Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372-5.
Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557-64.
Donati AR, Shokur S, Morya E, Campos DS, Moioli RC, Gitti CM, et al. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Scientific reports. 2016;6:30383.
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123 Pt 3:572-84.
Cortes M, Thickbroom GW, Valls-Sole J, Pascual-Leone A, Edwards DJ. Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol. 2011;122(11):2254-9.
Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 2012;22(24):2355-61.
Jackson A, Zimmermann JB. Neural interfaces for the brain and spinal cord--restoring motor function. Nature reviews Neurology. 2012;8(12):690-9.
Lequerica AH, Kortte K. Therapeutic engagement: a proposed model of engagement in medical rehabilitation. Am J Phys Med Rehabil. 2010;89(5):415-22.
Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45(2):229-40.
Putrino D, Zanders H, Hamilton T, Rykman A, Lee P, Edwards DJ. Patient Engagement Is Related to Impairment Reduction During Digital Game-Based Therapy in Stroke. Games for health journal. 2017;6(5):295-302.
Putrino D. Telerehabilitation and emerging virtual reality approaches to stroke rehabilitation. Curr Opin Neurol. 2014;27(6):631-6.
Koenig A, Omlin X, Bergmann J, Zimmerli L, Bolliger M, Muller F, et al. Controlling patient participation during robot-assisted gait training. Journal of neuroengineering and rehabilitation. 2011;8:14.
Novak D, Nagle A, Keller U, Riener R. Increasing motivation in robot-aided arm rehabilitation with competitive and cooperative gameplay. Journal of neuroengineering and rehabilitation. 2014;11:64.
Novak D, Nagle A, Riener R. Can two-player games increase motivation in rehabilitation robotics? Proceedings of the 2014 ACM/IEEE international conference on Human-robot interaction; Bielefeld, Germany. 2559658: ACM; 2014. p. 447-54.
Brutsch K, Schuler T, Koenig A, Zimmerli L, Koeneke SM, Lunenburger L, et al. Influence of virtual reality soccer game on walking performance in robotic assisted gait training for children. Journal of neuroengineering and rehabilitation. 2010;7:15.
Brutsch K, Koenig A, Zimmerli L, Merillat-Koeneke S, Riener R, Jancke L, et al. Virtual reality for enhancement of robot-assisted gait training in children with central gait disorders. J Rehabil Med. 2011;43(6):493-9.
Schuler T, Brutsch K, Muller R, van Hedel HJ, Meyer-Heim A. Virtual realities as motivational tools for robotic assisted gait training in children: A surface electromyography study. NeuroRehabilitation. 2011;28(4):401-11.
Zimmerli L, Jacky M, Lunenburger L, Riener R, Bolliger M. Increasing patient engagement during virtual reality-based motor rehabilitation. Arch Phys Med Rehabil. 2013;94(9):1737-46.
Bergmann J, Krewer C, Bauer P, Koenig A, Riener R, Muller F. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: a pilot randomized controlled trial. Eur J Phys Rehabil Med. 2017
Author information
Authors and Affiliations
Corresponding author
Additional information
SCI Special Issue 2018: Current and Emerging Therapeutic Approaches for Spinal Cord Injury
Electronic Supplementary Material
ESM 1
(PDF 1123 kb)
Rights and permissions
About this article
Cite this article
Mekki, M., Delgado, A.D., Fry, A. et al. Robotic Rehabilitation and Spinal Cord Injury: a Narrative Review. Neurotherapeutics 15, 604–617 (2018). https://doi.org/10.1007/s13311-018-0642-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-018-0642-3